Draw The Bonddot Lewis Diagram Of Pbr3
Draw The Bonddot Lewis Diagram Of Pbr3 - #2 mention lone pairs on the atoms. While drawing any lewis structure the below points should be follows: Lewis structure of pbr3 contains three single bonds between the phosphorus (p) atom and each bromine (br) atom. Web these diagrams are helpful because they allow us to show how atoms are connected, and when coupled with valence shell electron repulsion theory (vsepr), we can use lewis structures to predict the shape of the molecule. Web in the pbr 3 lewis structure, there are three single bonds around the phosphorus atom, with three bromine atoms attached to it. Here, we work towards sketching a skeleton diagram of the molecule with atoms represented by their symbols, valence electrons represented by dots, and bonds represented by straight lines. Pbr3 has 26 valence electrons of which bromine atoms can consume eight each in bonding pairs and two electrons in lone pairs on phosphorus. #4 calculate formal charge and check stability (if octet is already completed on central atom) let’s one by one discuss each step in detail. Part 3 (1 pt) what is the molecular geometry of pbr3? In the lewis structure for pbr 3 there are a total of 26 valence electrons. A lewis structure is a way to show how atoms share electrons when they form a molecule. The valence electrons of atoms form bonds, which are represented by straight lines. Part 3 (1 point) what is the molecular geometry of pbr3 ? Web draw the lewis structure of pbr3. Draw a lewis electron dot diagram for an atom or a. Select the element with lowest electronegativity for central position in structure. Each bromine atom has three lone pairs, and the phosphorus atom has one lone pair. Represent shared electron pairs (bonds) with dashes. Draw a lewis electron dot diagram for an atom or a monatomic ion. So, if you are ready to go with these 6 simple steps, then let’s. Use arrows to move dots (electrons) to fullfill octet rule 4. The valence electrons are the electrons in the outermost shell. With two bonding pairs and two lone pairs, the oxygen atom has now completed its octet. In order to find the total valence electrons in a pbr3 molecule, first of all you should know the valence electrons. For the. Use arrows to move dots (electrons) to fullfill octet rule 4. Web since the geometry is asymmetrical, the molecule will be polar. Draw lewis dot models of your elements involved near each other 2. Web hello guys!we are back with yet another video to help you determine the lewis structure of pbr3 or phosphorus bromide. Web steps of drawing pbr3. Include all the lone pairs. In order to find the total valence electrons in a pbr3 molecule, first of all you should know the valence electrons. Lewis dot structures or lewis structures are the diagrams that help to understand the bonding of atoms along with the lone pairs present in the molecule. In the pbr 3 lewis structure phosphorus (p). Web this was formulated by gilbert n lewis and stands for a diagrammatic representation of bonds and valence electrons of a chemical molecule. The valence electrons are the electrons in the outermost shell. Web these diagrams are helpful because they allow us to show how atoms are connected, and when coupled with valence shell electron repulsion theory (vsepr), we can. In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. Draw lewis dot models of your elements involved near each other 2. Use arrows to move dots (electrons) to fullfill octet rule 4. 100% (40 ratings) share share. Web we can illustrate the formation of a water molecule from two hydrogen atoms and an oxygen. Three pairs will be used in the chemical bonds between the p and br. In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. 100% (40 ratings) share share. Part 4 (1 point) which of the following best describes the bond angles in pbr3 ?. Do bonding between all the elements present in structure. Pbr3 has 26 valence electrons of which bromine atoms can consume eight each in bonding pairs and two electrons in lone pairs on phosphorus. Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. Lewis dot structures, also known as lewis structures, are diagrams that enable us to understand the bonding of atoms as well. Lewis dot structures, also known as lewis structures, are diagrams that enable us to understand the bonding of atoms as well as the lone pairs present in a molecule. While drawing any lewis structure the below points should be follows: Lewis dot structures or lewis structures are the diagrams that help to understand the bonding of atoms along with the. Phosphorus is in the 15th group of the periodic table, and bromine is in the 17th group. Three pairs will be used in the chemical bonds between the p and br. 100% (40 ratings) share share. #1 draw a rough skeleton structure. Draw lewis dot models of your elements involved near each other 2. The total available valence electrons are 26, calculated as 5 + 3 (7). Predict how many more electrons each needs 3. Web draw the lewis structure of pbr3. Web these diagrams are helpful because they allow us to show how atoms are connected, and when coupled with valence shell electron repulsion theory (vsepr), we can use lewis structures to predict the shape of the molecule. Web in the pbr 3 lewis structure, there are three single bonds around the phosphorus atom, with three bromine atoms attached to it. Web lewis structures for covalent bonds 1. Each bromine atom has three lone pairs, and the phosphorus atom has one lone pair. Include all the lone pairs. Draw a lewis electron dot diagram for an atom or a monatomic ion. Use arrows to move dots (electrons) to fullfill octet rule 4. In the pbr 3 lewis structure phosphorus (p) is the least electronegative so it goes in the center.
Pbr3 Lewis Structure Shape Draw Easy

Draw the lewis structure of PBr3 NO21 Brainly.in

PBr3 Molecular Geometry / Shape and Bond Angles YouTube

PBr3 Lewis Structure (Phosphorus Tribromide) YouTube

PBr3 Lewis Structure, Molecular Geometry, Polarity, and Hybridization
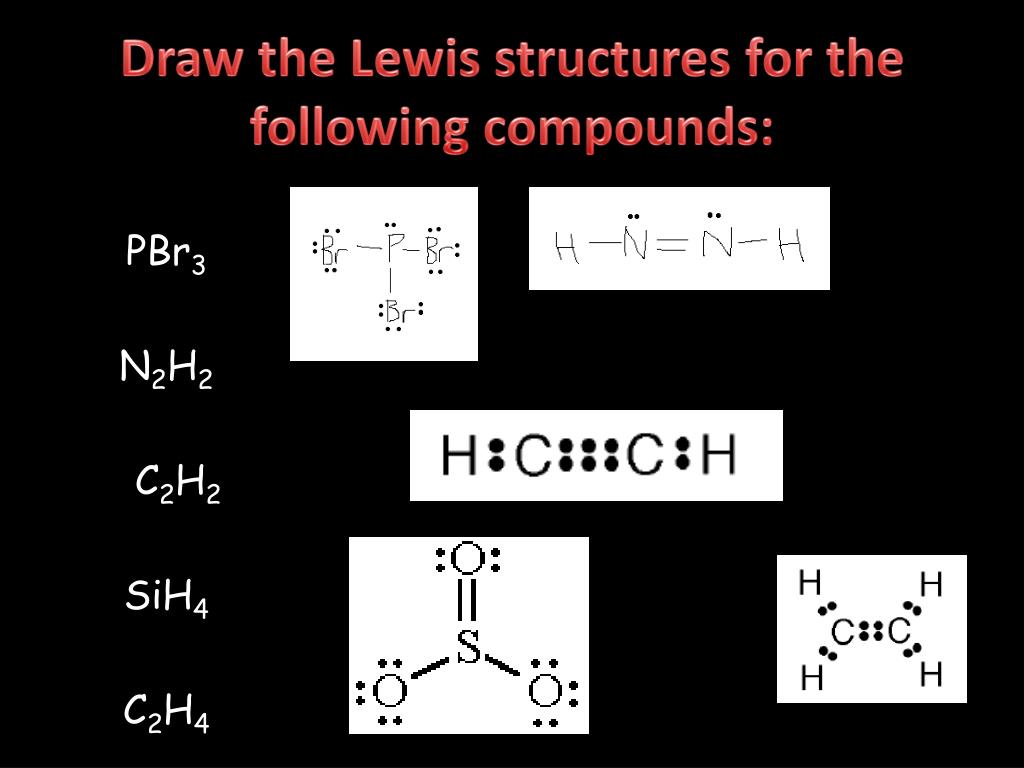
PPT Lewis Dot Structure PowerPoint Presentation, free download ID

Draw The Lewis Structure Of Pbr3 Draw Easy

Draw the Lewis Structure for the Phosphorus Tribromide Pbr3 Molecule.
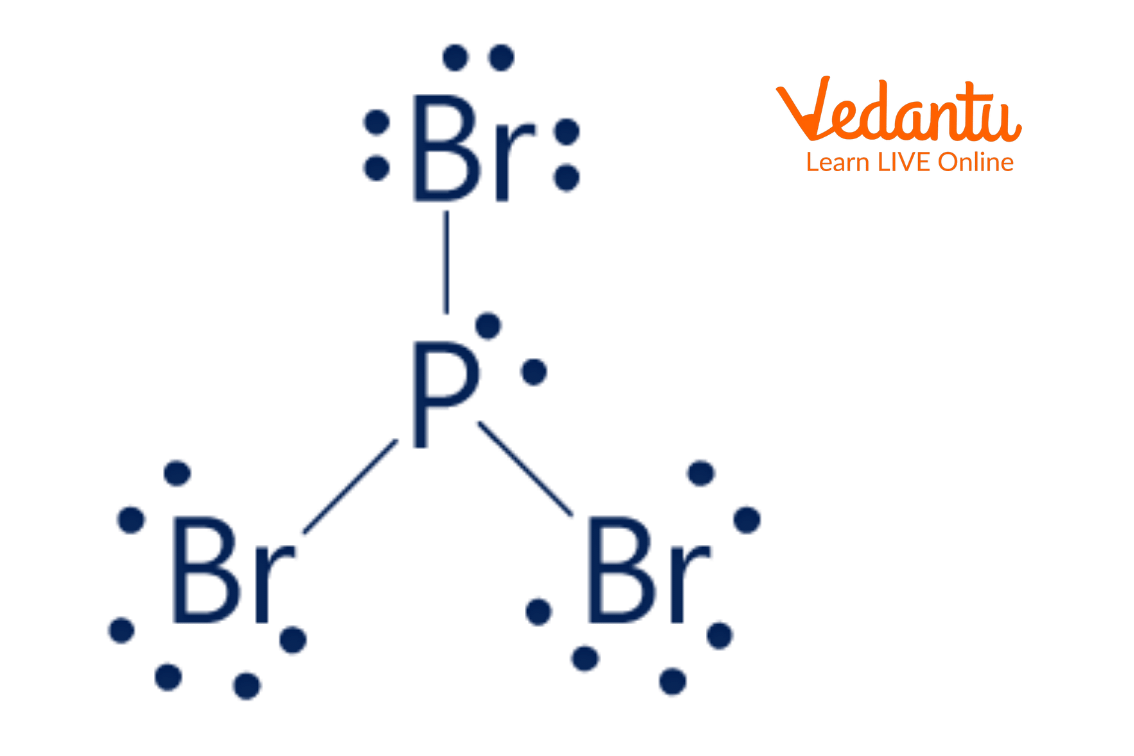
Lewis Structure of PBr3 Learn Important Terms and Concepts
Draw The Lewis Structure Of Pbr3 Draw Easy
#2 Mention Lone Pairs On The Atoms.
Get The Free Lewis Structure Finder Widget For Your Website, Blog, Wordpress, Blogger, Or Igoogle.
In Order To Find The Total Valence Electrons In A Pbr3 Molecule, First Of All You Should Know The Valence Electrons.
There Are 2 Steps To Solve This One.
Related Post: