Draw The Electron Configuration For A Neutral Atom Of Boron
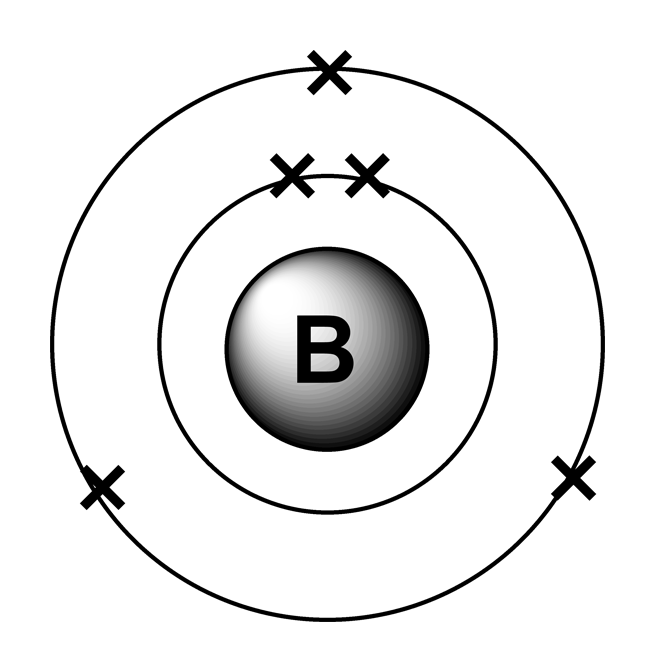
Draw The Electron Configuration For A Neutral Atom Of Boron - The noble gas configuration is [he]2s22p1. Web an electron configuration diagram is a model that depicts the position of electrons as they orbit the nucleus of an atom. At carbon, with z = 6 and six electrons, we are faced with a choice. We fill both the 1 s and 2 s orbitals to achieve a. In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. 5 valence electrons/atom × 1 atom = 5 o: Web electron configurationthe arrangements of electrons above the last (closed shell) noble gas. Draw a lewis electron dot diagram for an atom or a monatomic ion. Web what is the electron configuration of: 1s 2 2s 2 2p 1. A neutral atom has the. Boron has atomic number 5, which means that it has 5 protons in its atomic nuclei. Web 19 rows the electron configuration of boron is: Web draw the electron configuration for a neutral atom of boron. At carbon, with z = 6 and six electrons, we are faced with a choice. At carbon, with z = 6 and six electrons, we are faced with a choice. This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element you choose. Web the electron configuration of boron is 1 s2 2 s2 2 p1: 1s 2 2s 2 2p 1: 1s 2 2s 2 2p. Web this electron configuration is written as 1 s2 2 s1. Web 19 rows the electron configuration of boron is: Draw a lewis electron dot diagram for an atom or a monatomic ion. Should the sixth electron be placed in the same 2. The noble gas configuration is [he]2s22p1. 1s 2 2s 2 2p 1. Using only the periodic table; The next element is beryllium, with z = 4 and four electrons. The noble gas configuration is [he]2s22p1. This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element you choose. Electron configuration of carbon (c) [he] 2s 2 2p 2: 5 valence electrons/atom × 1 atom = 5 o: Web the electron configuration of boron is 1 s2 2 s2 2 p1: Identify and explain exceptions to predicted electron configurations for atoms and ions. 1s 2 2s 2 2p 2: Boron has atomic number 5, which means that it has 5 protons in its atomic nuclei. 1s 2 2s 2 2p 2: 1s 2 2s 2 2p 1. We fill both the 1 s and 2 s orbitals to achieve a. In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. At carbon, with z = 6 and six electrons, we are faced with a choice. The next element is beryllium, with z = 4 and four electrons. Web an electron configuration diagram is a model that depicts the position of electrons as they orbit. Identify and explain exceptions to predicted electron configurations for atoms and ions. 6 valence electron/atom × 1 atom = 6 + −1 electron (positive charge) = −1 ¯ = 10 valence electrons no + n: The next element is beryllium, with z = 4 and four electrons. A neutral atom has the. Boron has atomic number 5, which means that. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web a lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or a lewis structure) is a representation of the valence electrons of an atom that uses. Web this electron configuration is written as 1 s2 2 s1. 1s 2 2s. Using only the periodic table; Now, to draw the bohr model for boron we first need to identify the number of different atomic particles that this. At carbon, with z = 6 and six electrons, we are faced with a choice. The noble gas configuration is [he]2s22p1. Energy 1 х 5 this problem has been solved! Now, to draw the bohr model for boron we first need to identify the number of different atomic particles that this. Web this electron configuration is written as 1 s2 2 s1. Web an electron configuration diagram is a model that depicts the position of electrons as they orbit the nucleus of an atom. Energy 1 х 5 this problem has been solved! Web the electron configuration of boron is 1s 2 2s 2 2p 1: 1s 2 2s 2 2p 1. Web 19 rows the electron configuration of boron is: Using only the periodic table; Web the electron configuration of boron is 1 s2 2 s2 2 p1: The next element is beryllium, with z = 4 and four electrons. 1s 2 2s 2 2p 2: In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. Web intro to electron configurations; Draw a lewis electron dot diagram for an atom or a monatomic ion. Boron has one electron pair in the 1 s orbital, one electron pair in the 2 s orbital, and one electron in the 2 p orbital. Web a lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or a lewis structure) is a representation of the valence electrons of an atom that uses.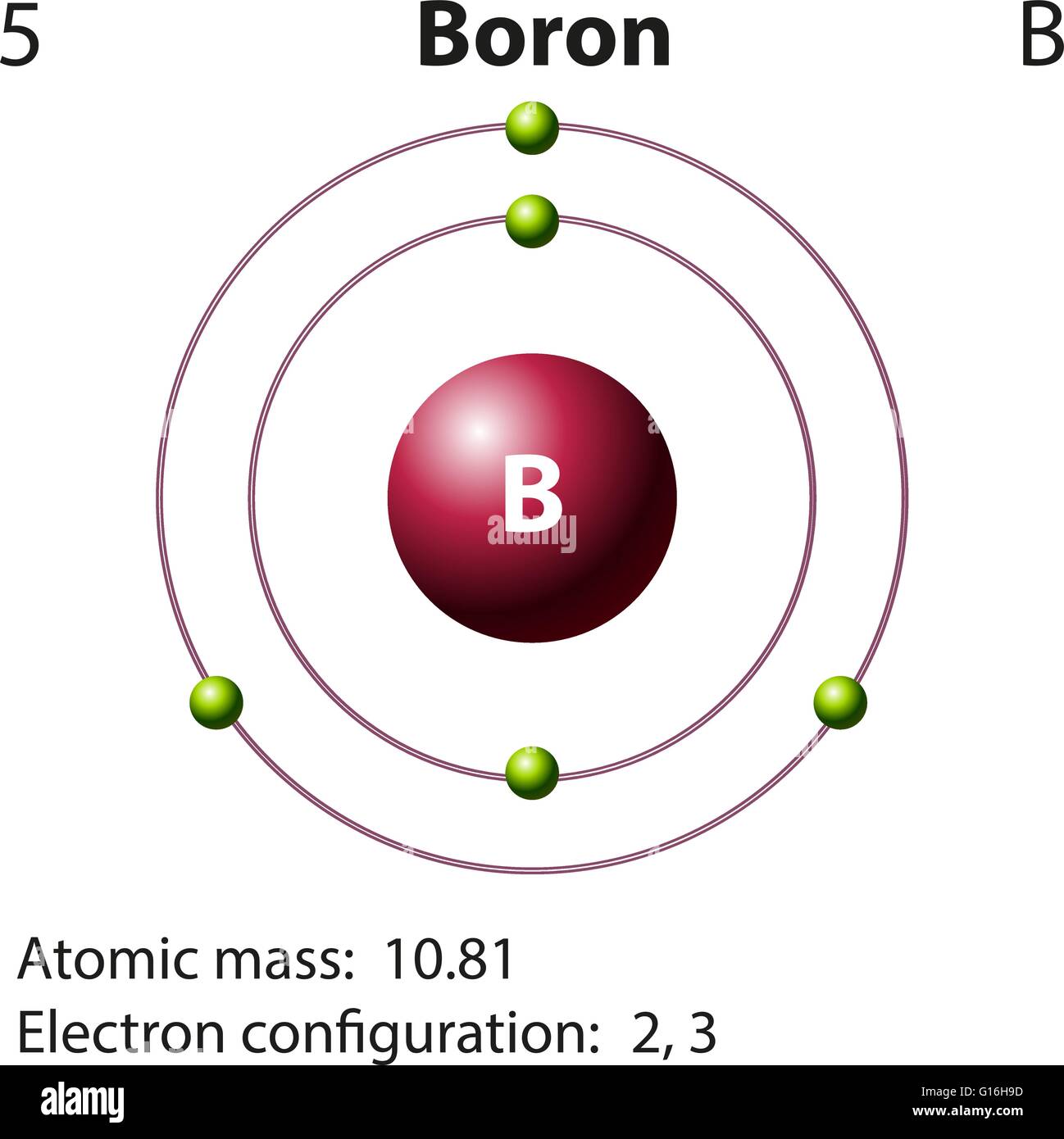
Diagram representation of the element boron illustration Stock Vector
Boron Element Model
Electron arrangements

Boron Electron Configuration YouTube

Boron, atomic structure Stock Image C018/3686 Science Photo Library
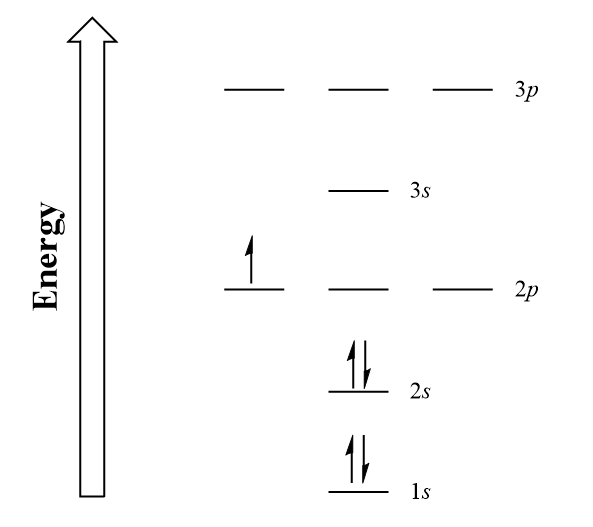
Boron_electron_configuration_energy_diagram Introductory Chemistry
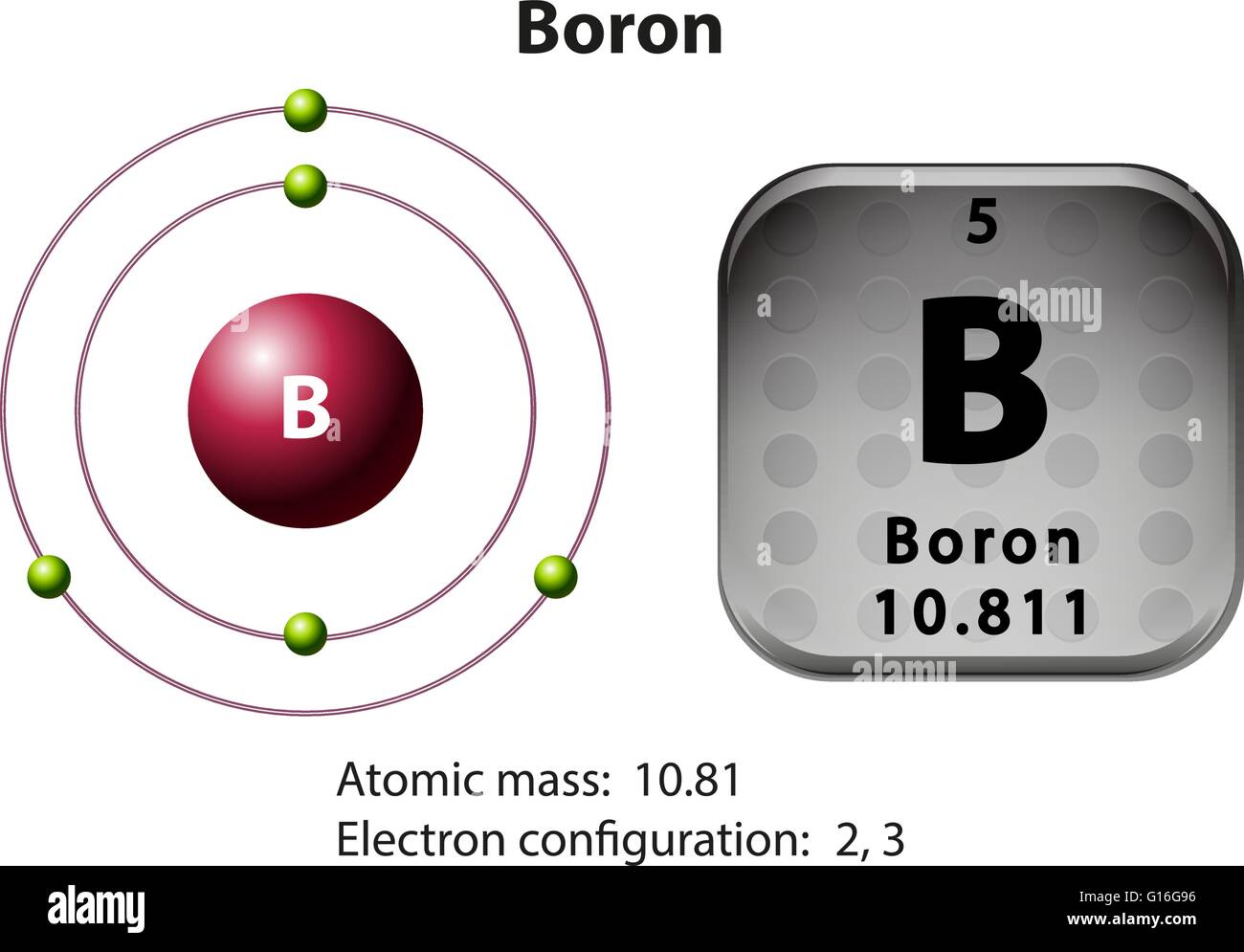
Symbol and electron diagram Boron illustration Stock Vector Image & Art
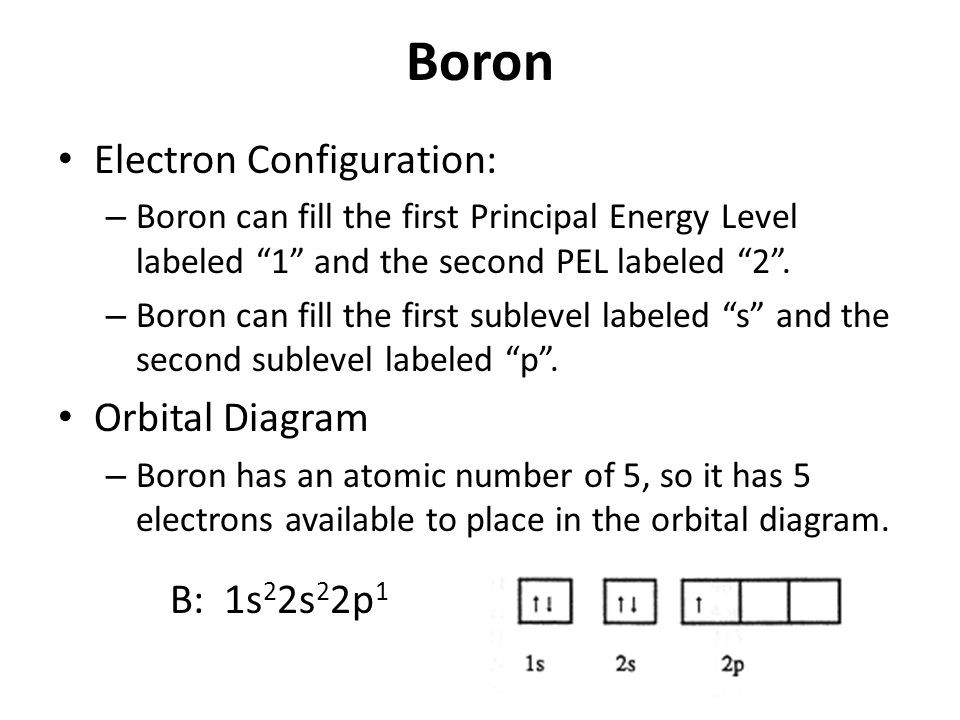
How To Find The Boron Electron Configuration (B)
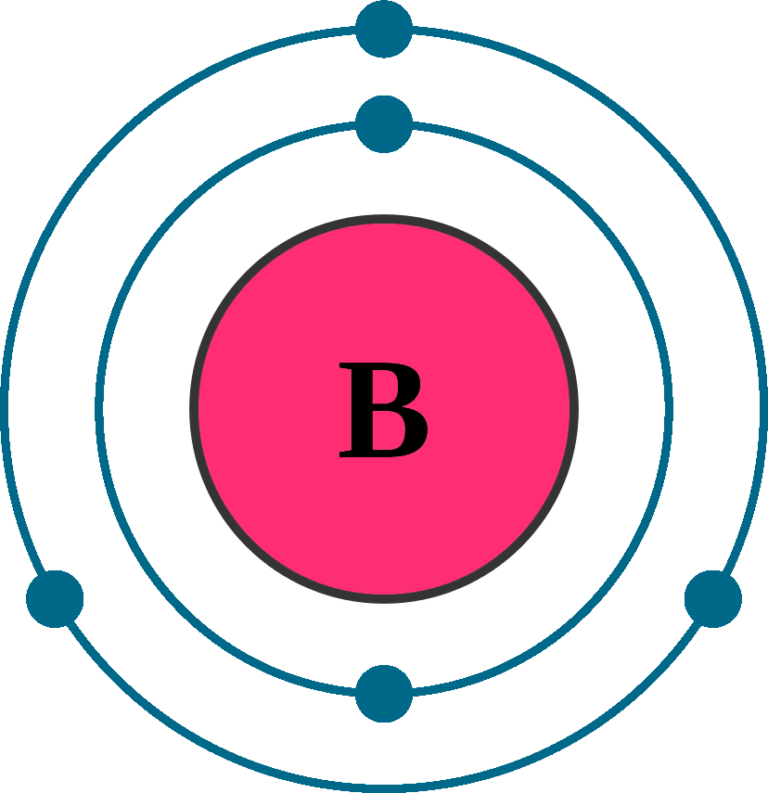
Boron Element With Reaction, Properties, Uses, & Price Periodic Table

Boron Electron Configuration And Full Orbital Diagram
1S 2 2S 2 2P 1:
The Noble Gas Configuration Is [He]2S22P1.
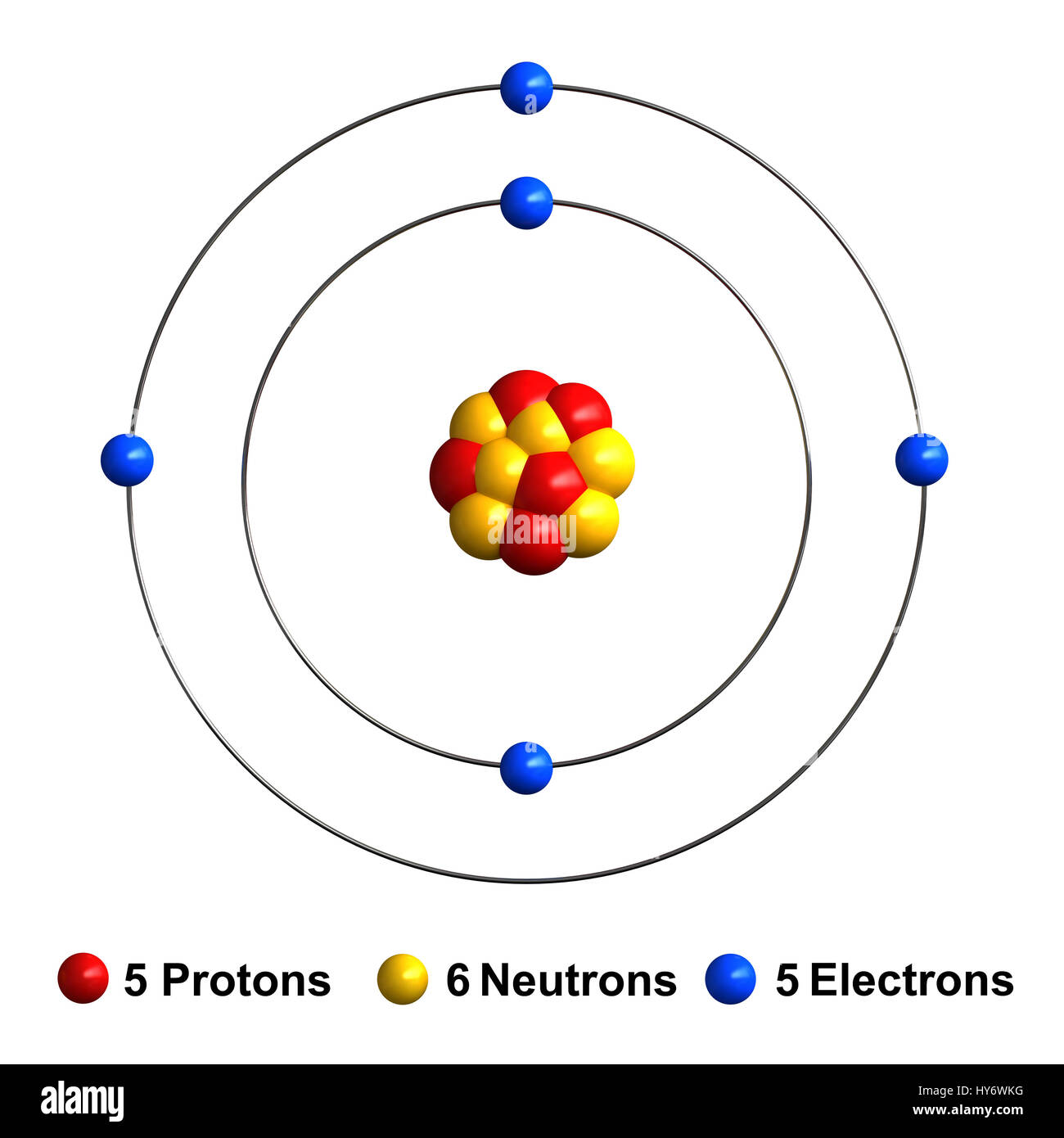
Boron Has Atomic Number 5, Which Means That It Has 5 Protons In Its Atomic Nuclei.
Web Electron Configuration Of Boron (B) [He] 2S 2 2P 1:
Related Post: