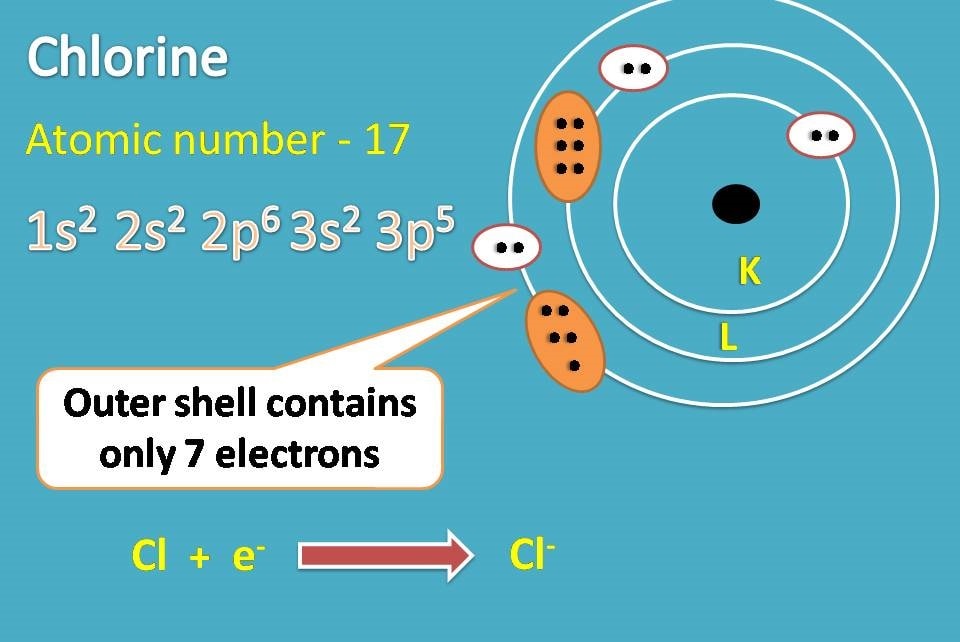
Draw The Electron Configuration For A Neutral Atom Of Chlorine
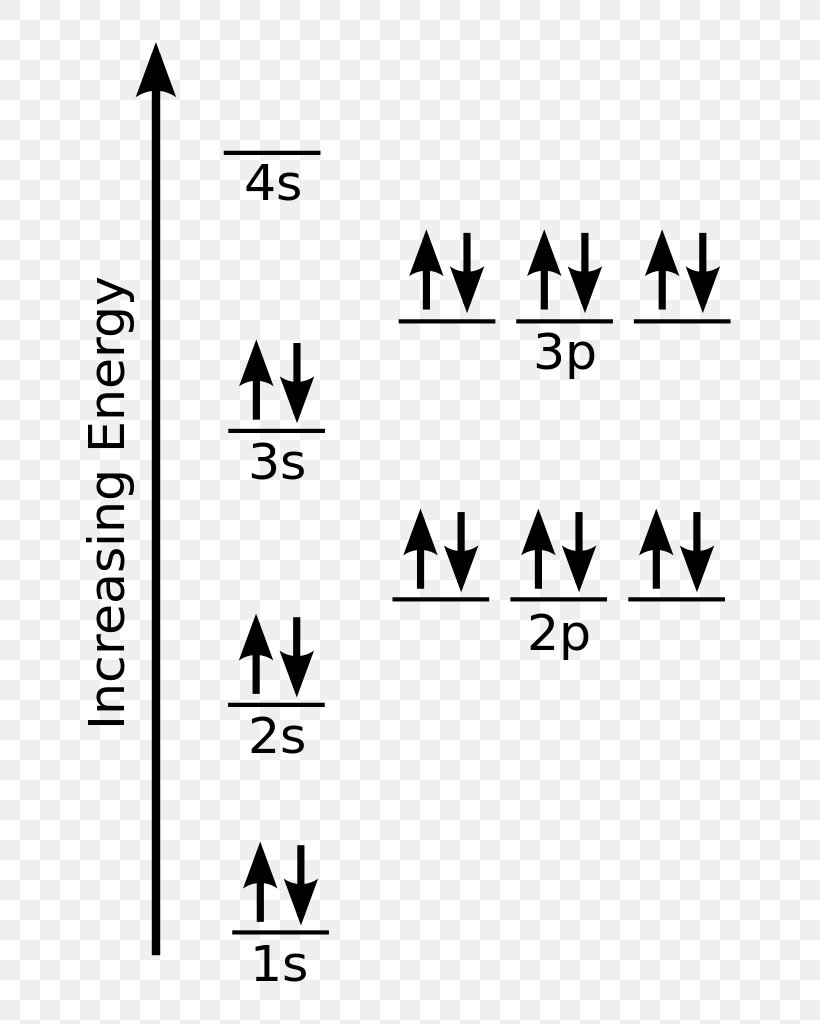
Draw The Electron Configuration For A Neutral Atom Of Chlorine - So, the neutral atom of chlorine contains 17 electrons. Web using s p d f notation, what is the electron configuration for a neutral atom of beryllium? What is its valence electron configuration?. 1 s 2 2 s 1 2 p 1. Using figure \(\pageindex{2}\) as your guide, write the electron configuration of a neutral. Web the electron configuration for a chloride ion is: A review of general chemistry atomic structure. The atomic number of chlorine, cl = 17. 1 s2 2 s2 2 p6 3 s2 3. 1 s 2 2 s 1 2 p 1. 1 s2 2 s2 2 p6 3 s2 3. Therefore, its ground state electronic configuration can be written as 1s 2 2s 2 2p 6 3s 2 3p 5. The configuration notation provides an easy. 1 s 2 2 s 2. Web therefore the chlorine electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 5. Therefore, its ground state electronic configuration can be written as 1s 2 2s 2 2p 6 3s 2 3p 5. Web what is the electron configuration of: The element atomic number and name are listed. Web the neutral atom chlorine (z=17), for instance has 17 electrons. Web the electron configuration for a chloride ion is: Electronic configuration of chlorine atoms. 1s², 2s², 2p⁶, 3s², 3p⁶ so, the final answer is: The element atomic number and name are listed. Therefore, its ground state electronic configuration can be written as 1s 2 2s 2 2p 6 3s 2 3p 5. So, the neutral atom of chlorine contains 17 electrons. Web the electron configuration for a chloride ion is: Electronic configuration of chlorine atoms. What is the electron configuration of a neutral chlorine atom? Web if a neutral atom of chlorine picks up an electron, well, the electron would add right in here. Web draw an orbital diagram and use it to derive the electron configuration of chlorine, z =. Using figure \(\pageindex{2}\) as your guide, write the electron configuration of a neutral. Electronic configuration of chlorine atoms. Web the electron configuration for a chloride ion is: Using figure \(\pageindex{2}\) as your guide, write the electron configuration of a neutral. A review of general chemistry atomic structure. Electronic configuration of chlorine atoms. $\boxed {1s^2, 2s^2, 2p^6, 3s^2, 3p^5}$ chloride ion: Web the final ring or shell of electrons contains the typical number of valence electrons for an atom of that element. The configuration notation provides an easy. Using figure \(\pageindex{2}\) as your guide, write the electron configuration of a neutral. Web draw an orbital diagram and use it to derive the electron configuration of chlorine, z = 17. Web using s p d f notation, what is the electron configuration for a neutral atom of beryllium? The element atomic number and name are listed. Web if a neutral atom of chlorine picks up an electron, well, the electron would add. Web draw an orbital diagram and use it to derive the electron configuration of chlorine, z = 17. Electronic configuration of chlorine atoms. 1 s 2 2 s 1 2 p 1. $\boxed {1s^2, 2s^2, 2p^6, 3s^2, 3p^5}$ chloride ion: 1 s2 2 s2 2 p6 3 s2 3. Web therefore the chlorine electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 5. Web by kirsty patterson 6 september 2021. View the full answer step 2. $\boxed {1s^2, 2s^2, 2p^6, 3s^2, 3p^5}$ chloride ion: Web using s p d f notation, what is the electron configuration for a neutral atom of beryllium? Using figure \(\pageindex{2}\) as your guide, write the electron configuration of a neutral. Aufbau principle, hund's rule and the pauli. Web the electron configuration for a chloride ion is: The electron configuration of an atomic species (neutral or ionic) allows us to understand the shape and energy of its electrons. The configuration notation provides an easy. Web by kirsty patterson 6 september 2021. View the full answer step 2. 1 s 2 2 s 1 2 p 1. Web what is the electron configuration of: What is the electron configuration for a neutral chlorine atom? Aufbau principle, hund's rule and the pauli. What is the electron configuration of a neutral chlorine atom? Web there are a set of general rules that are used to figure out the electron configuration of an atomic species: 1 s 2 2 s 2. 1 s2 2 s2 2 p6 3 s2 3. The configuration notation provides an easy. Electronic configuration of chlorine atoms. 1s², 2s², 2p⁶, 3s², 3p⁶ so, the final answer is: The element atomic number and name are listed. A review of general chemistry atomic structure. Electronic configuration of chlorine atoms.:max_bytes(150000):strip_icc()/chlorineatom-58b602515f9b5860464c5c02.jpg)
Atom Diagrams Electron Configurations of the Elements

Electron Configuration For Chlorine
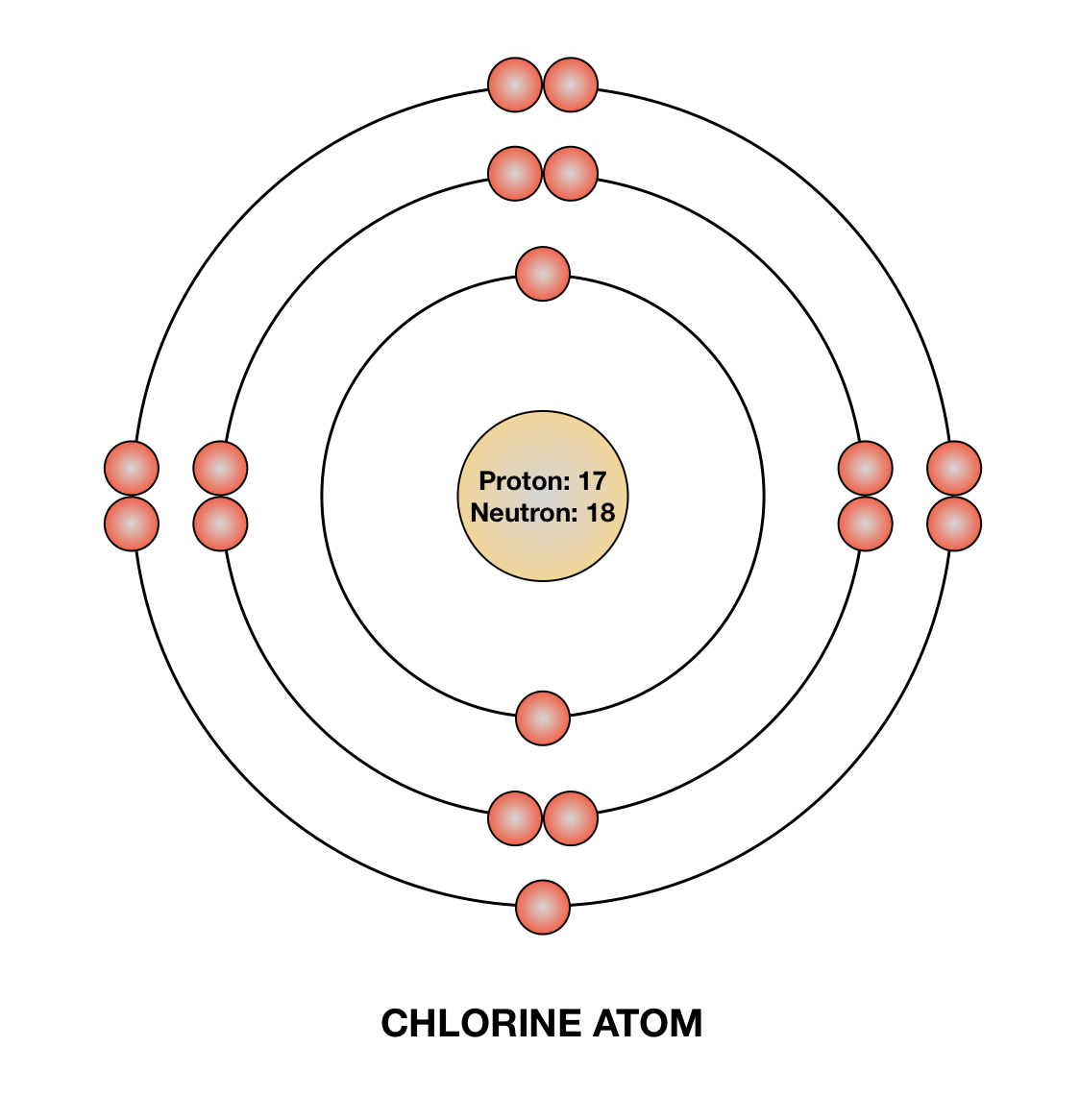
Draw a Bohr diagram of chlorine. Quizlet

Atomic Diagram Of Chlorine

Chlorine Electron Configuration YouTube

draw atomic structure of chlorine Brainly.in
Lewis dot structure How to write?
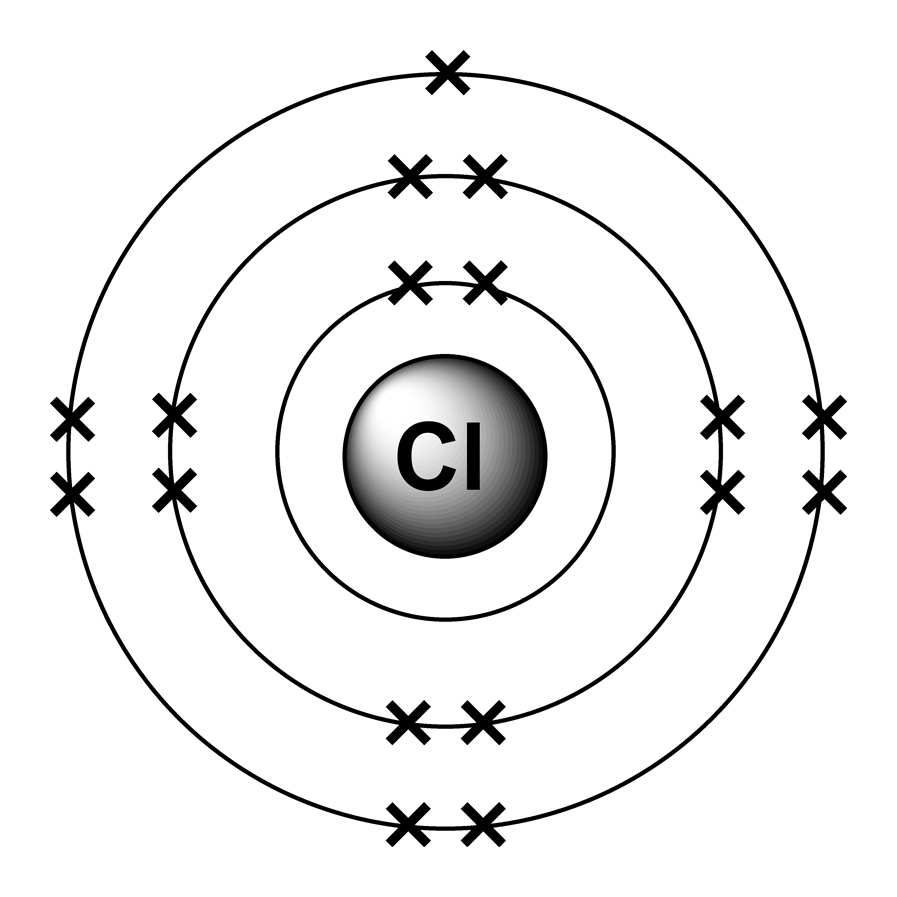
Electron arrangements
Chlorine Periodic Table Electron Configuration Elcho Table
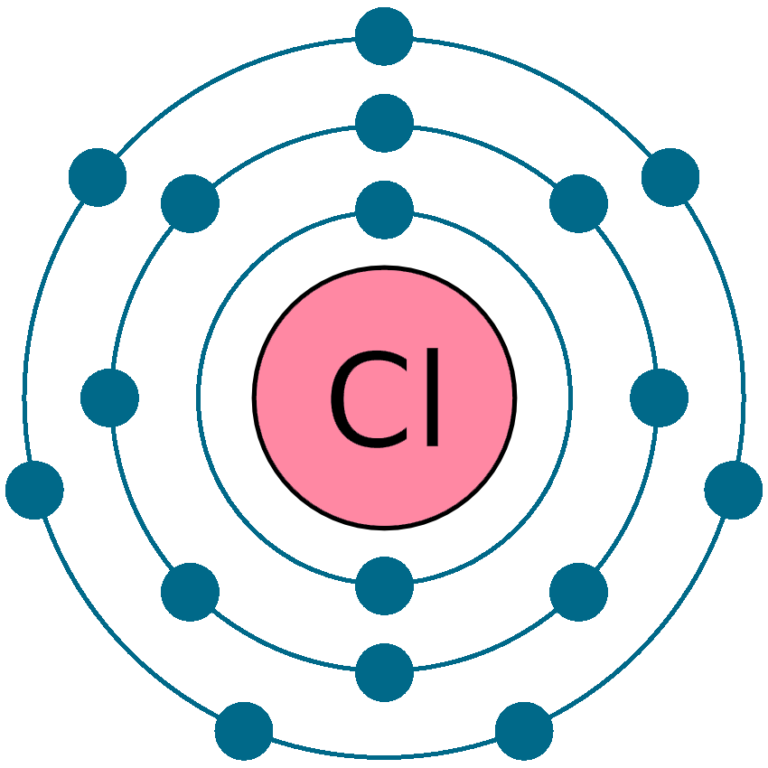
Chlorine Cl (Element 17) of Periodic Table NewtonDesk
Web The Electron Configuration For A Chloride Ion Is:
So, The Neutral Atom Of Chlorine Contains 17 Electrons.
What Is Its Valence Electron Configuration?.
Web Using S P D F Notation, What Is The Electron Configuration For A Neutral Atom Of Beryllium?
Related Post: