Draw The Lewis Dot Diagram For A Cation
Draw The Lewis Dot Diagram For A Cation - Likewise, they can be used to show the formation of anions from atoms, as shown here for chlorine and sulfur: Web now most people will represent this dot structure by putting brackets here. Post any question and get expert help quickly. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful. Web lewis symbols can be used to illustrate the formation of cations from atoms, as shown here for sodium and calcium: Web lewis electron dot diagrams use dots to represent valence electrons around an atomic symbol. The example is for the nitrate ion. Once we know how many. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. In section 4.7, we demonstrated that ions are formed by losing electrons to make cations, or by gaining electrons to form anions. Web lewis electron dot diagrams use dots to represent valence electrons around an atomic symbol. So we're going to a lot more examples for drawing dot structures in the next several videos, and see how drawing dot structures allows you to predict the shapes of different molecules. There are 2 steps to solve this one. Get the free lewis structure. Here’s how to approach this question. Lewis electron dot diagrams for ions have less (for cations) or more (for anions) dots than the corresponding atom. Most atoms do not have eight electrons in their valence electron shell. For the k+ structure use the periodic table to find the total number of valence electrons for k. Draw a lewis electron dot. To draw lewis structures for molecules and polyatomic ions with one central atom. Most atoms do not have eight electrons in their valence electron shell. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. View the full answer answer. The lewis dot diagram for a cl a. Draw the lewis dot diagram for a cl2+ cation. For the k+ structure use the periodic table to find the total number of valence electrons for k. Send feedback | visit wolfram|alpha. Lewis structures show all of the valence electrons in an atom or molecule. Draw lewis structures for ionic compounds. The astute reader may have noticed something: Many of the ions that form have eight electrons in their valence shell. In section 4.7, we demonstrated that ions are formed by losing electrons to make cations, or by gaining electrons to form anions. Lewis electron dot diagrams for ions have less (for cations) or more (for anions) dots than the corresponding. Once we know how many. Web here are the steps to draw a lewis structure. Using lewis structures to show valence electrons. The example is for the nitrate ion. Send feedback | visit wolfram|alpha. Likewise, they can be used to show the formation of anions from atoms, as shown here for chlorine and sulfur: However, these structures are helpful in understanding the bonding and valence electron configurations of different atoms and molecules. Find more chemistry widgets in wolfram|alpha. So there's your xenon pentafluoride cation. For the na+ structure use the periodic table to find. How to draw a lewis structure. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful. The example is for the nitrate ion. Draw a lewis electron dot diagram for an atom or a monatomic ion. Using lewis structures to show valence electrons. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful. However, these structures are helpful in understanding the bonding and valence electron configurations of different atoms and molecules. When constructing a lewis diagram, keep in mind the octet rule, which refers to the tendency. So there's your xenon pentafluoride cation.. Identify the atomic number of arsenic (as) which is 33, and understand its electronic configuration [ar] 3d^10 4s^2 4p^3 signifies a valence shell of 5. A lewis structure is a way to show how atoms share electrons when they form a molecule. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would. Lewis electron dot diagrams for ions have less (for cations) or more (for anions) dots than the corresponding atom. Using lewis structures to show valence electrons. For the na+ structure use the periodic table to find the total number of valence electrons. And putting a positive charge outside of it. The octet rule refers to the tendency of atoms to. How to draw a lewis structure. The diagram is also called a lewis dot diagram, lewis dot formula, or electron dot diagram. Draw a lewis electron dot diagram for an atom or a monatomic ion. Web here are the steps to draw a lewis structure. Web lewis symbols can also be used to illustrate the formation of cations from atoms, as shown here for sodium and calcium: The example is for the nitrate ion. Lewis electron dot diagrams for ions have less (for cations) or more (for anions) dots than the corresponding atom. Web drawing lewis dot structures (also known as lewis structures or lewis diagrams) can be confusing, particularly for a beginning chemistry student. Send feedback | visit wolfram|alpha. There are 2 steps to solve this one. For the arseniclewis structure use the periodic.
How To Draw Lewis Structures A Step By Step Tutorial
How To Draw Lewis Dot Diagrams Simplereality27

Lewis Electron Dot Structure Calculator

Lewis Dot Structures of Atoms and Ions YouTube

How To Draw Lewis Dot Diagrams Simplereality27

How to Draw Lewis Dot Structure

Lewis Diagrams Made Easy How to Draw Lewis Dot Structures Watch
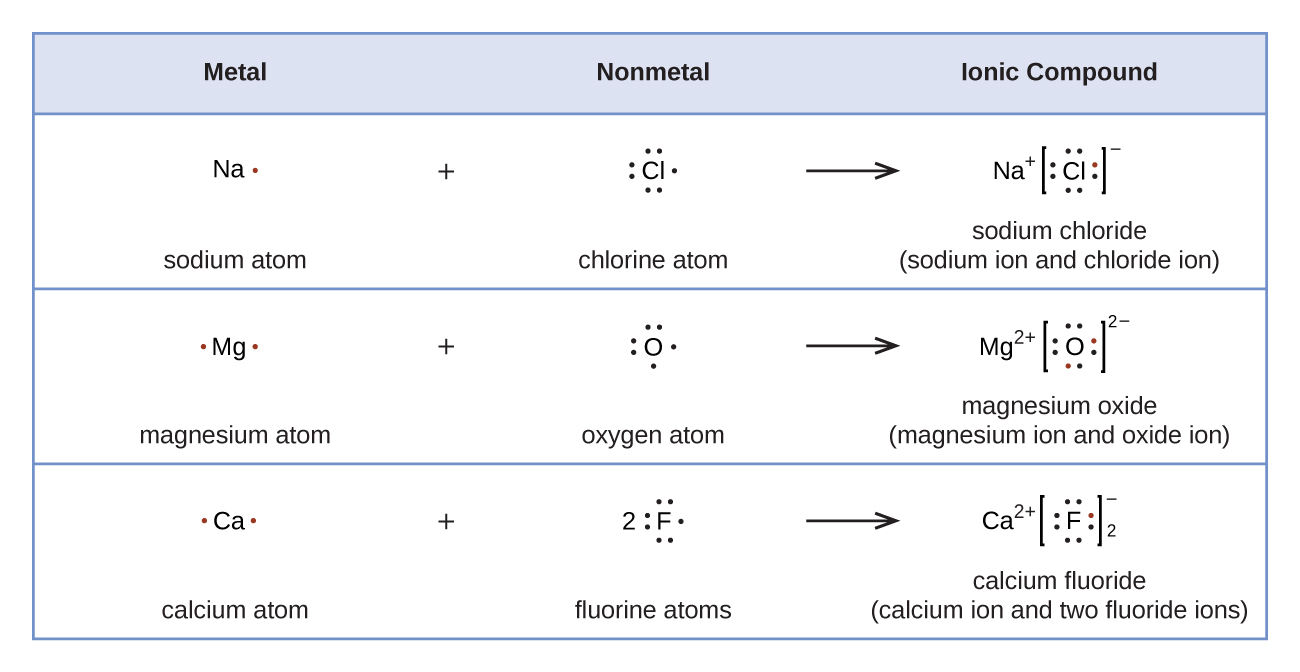
4.1 Lewis Dot Diagrams Chemistry LibreTexts

Lewis Structure Types

3 manières de dessiner une représentation de Lewis
140K Views 5 Years Ago.
For The K+ Structure Use The Periodic Table To Find The Total Number Of Valence Electrons For K.
When Constructing A Lewis Diagram, Keep In Mind The Octet Rule, Which Refers To The Tendency.
Web Draw The Lewis Dot Diagram For A As+ Cation.
Related Post: