Draw The Lewis Structure For Asf3
Draw The Lewis Structure For Asf3 - Web use these steps to correctly draw the asf 3 lewis structure: Find the total valence electrons in asf3 molecule. Web 28k views 10 years ago. Using lewis dot symbols to describe covalent bonding. #2 mark lone pairs on the atoms. (a) asf3 (b) ch3+ (c) brf3 (d) clo3− (e) xef2 (f) bro2−. Draw the lewis structure for bro2−. Web to draw lewis structures for molecules and polyatomic ions with one central atom. Draw the lewis structure for asf3. To use lewis dot symbols to explain the stoichiometry of a compound. #4 calculate formal charge and check stability (if octet is already completed on central atom) let’s one by one discuss each step in detail. Using lewis dot symbols to describe covalent bonding. Determine total electrons pairs as lone pairs and bonds. 2.draw the lewis structure for ch3+. This problem has been solved! Web the following procedure can be used to draw lewis structure for simple molecules. Draw the lewis structure for brf3. Ionic bonding typically occurs when it is easy for one atom to lose one or more electrons, and for another atom to gain one or more electrons. The valence electrons are the electrons in the. #3 calculate and mark formal. Using lewis dot symbols to describe covalent bonding. Find center atom and basic sketch. Lewis structure of asf3 contains three single bonds between the arsenic (as) atom and each fluorine (f) atom. Draw the lewis structure for clo3−. Find total number of electrons of the valance shells of arsenic and fluorine atoms. To use lewis dot symbols to explain the stoichiometry of a compound. Web steps of drawing asf3 lewis structure. Draw the molecule by placing atoms on the grid and connecting them with bonds. Find out by adding single, double or triple bonds and lone pairs to the central atom. It is also soluble in alcohols, ethers, and ammonia solutions. Asf3 is a colorless liquid made of one arsenic and three fluorine atoms. Find center atom and basic sketch. Asf3 exhibits a trigonal pyramidal geometry, with bond angles slightly less than 109.5° due to the lone pair. Draw the lewis structure for asf3. Web define covalent bond. Determine total electrons pairs as lone pairs and bonds. In order to find the total valence electrons in an asf3 molecule, first of all you should know the valence electrons. #3 calculate and mark formal charges on the atoms, if required. Find the total valence electrons in asf3 molecule. Draw the molecule by placing atoms on the grid and connecting. Explore molecule shapes by building molecules in 3d! Find total number of electrons of the valance shells of arsenic and fluorine atoms. Find out by adding single, double or triple bonds and lone pairs to the central atom. #1 first draw a rough sketch. Web 28k views 10 years ago. Web © 2023 google llc. However, some atoms will not give up or gain electrons easily. Send feedback | visit wolfram|alpha. Find the total valence electrons in asf3 molecule. Then, compare the model to real molecules! Lewis structures are diagrams used to show the valence electrons of atoms and the bonds that form between them. Web define covalent bond. Web here, i have explained 6 simple steps to draw the lewis dot structure of asf3 (along with images). By using the following steps, you can easily draw the lewis structure of asf 3. This problem has. Find total number of electrons of the valance shells of arsenic and fluorine atoms. Web define covalent bond. Lewis structure of asf3 contains three single bonds between the arsenic (as) atom and each fluorine (f) atom. Web here, i have explained 6 simple steps to draw the lewis dot structure of asf3 (along with images). Draw the lewis dot structure. Mark lone pairs on atoms. Let’s discuss each step in more detail. Explore molecule shapes by building molecules in 3d! Asf3 is a colorless liquid made of one arsenic and three fluorine atoms. Using lewis dot symbols to describe covalent bonding. The valence electrons are the electrons in the. Determine the total number of valence electrons in the molecule or ion. Web draw lewis structures depicting the bonding in simple molecules. Draw the lewis structure for xef2. Ionic bonding typically occurs when it is easy for one atom to lose one or more electrons, and for another atom to gain one or more electrons. The chemical formula asf3 represents arsenic trifluoride. Then, compare the model to real molecules! Web draw the most important lewis structure for asf3 and then answer the following questions. Web define covalent bond. Draw the lewis structure for asf3 draw the lewis dot structure. All other atoms are bonded directly to the central atom.
AsF3 Lewis Structure,Geometry,Hybridization5 Steps (Solved)

Arsenic Trifluoride Asf3 Chemspider
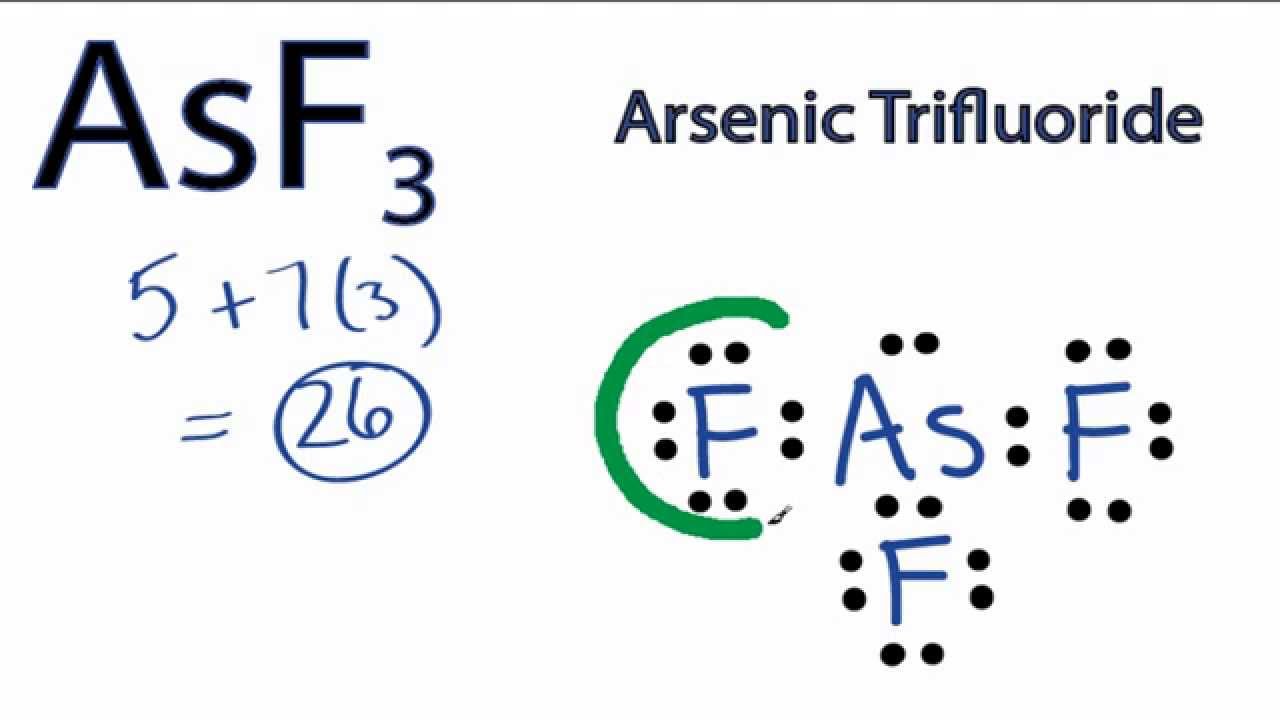
AsF3 Lewis Structure How to Draw the Lewis Structure for Arsenic

Draw the Lewis structure for AsF3 YouTube
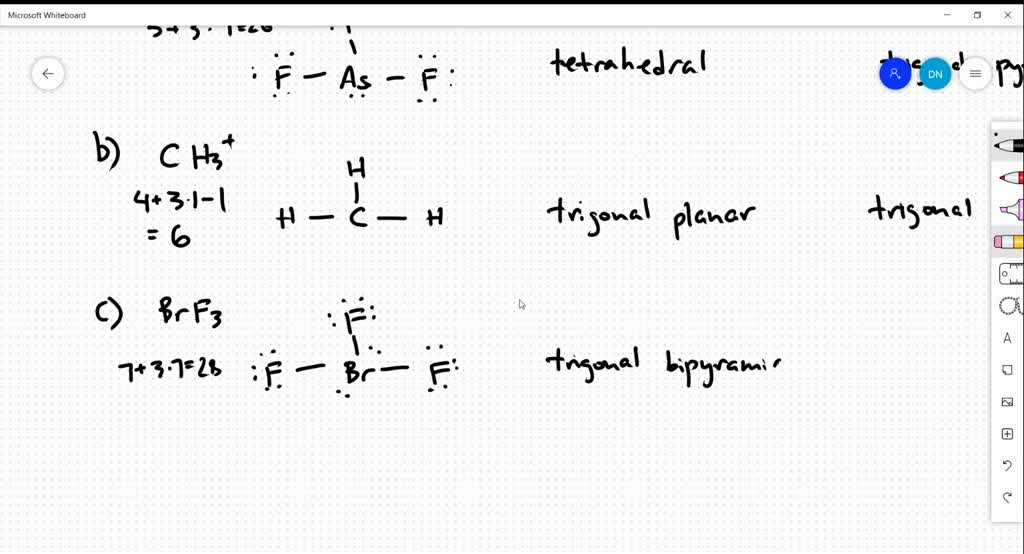
SOLVED Draw the Lewis structure for each of the following molecules or

How to draw AsF3 Lewis Structure? 4
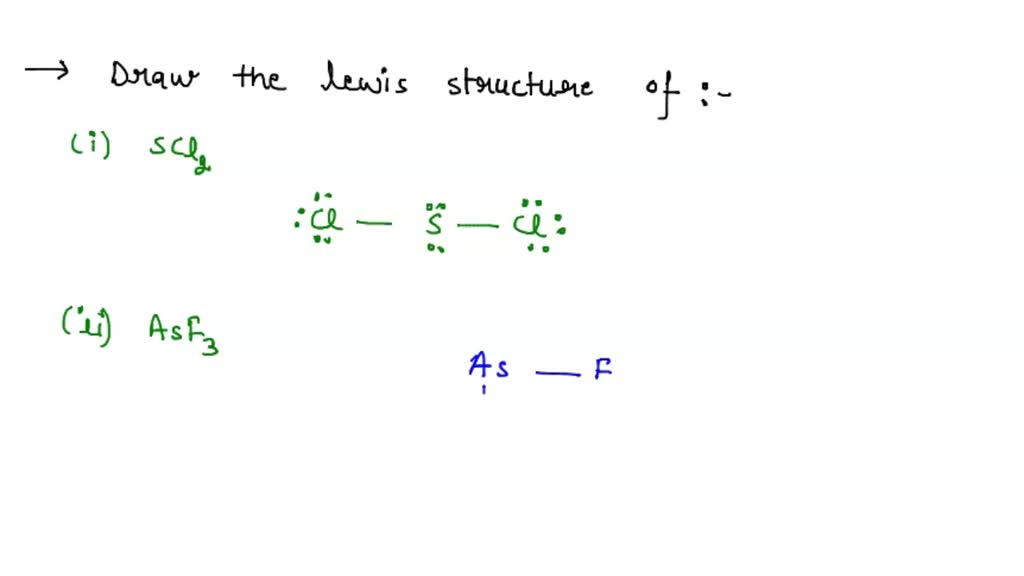
SOLVED 1) Draw the Lewis structure of A) SCl2 B) AsFr3 2) Using only

AsF3 (Arsenic trifluoride) Molecular Geometry, Bond Angles YouTube

How to draw AsF3 Lewis Structure? 3

AsF3 Lewis Structure (Arsenic Trifluoride) Lewis, Molecules, Electrons
Send Feedback | Visit Wolfram|Alpha.
In Order To Find The Total Valence Electrons In An Asf3 Molecule, First Of All You Should Know The Valence Electrons.
For The Asf3 Structure Use The Periodic Table To Find The Total.
It Is Also Soluble In Alcohols, Ethers, And Ammonia Solutions.
Related Post: