Draw The Lewis Structure For Brf3
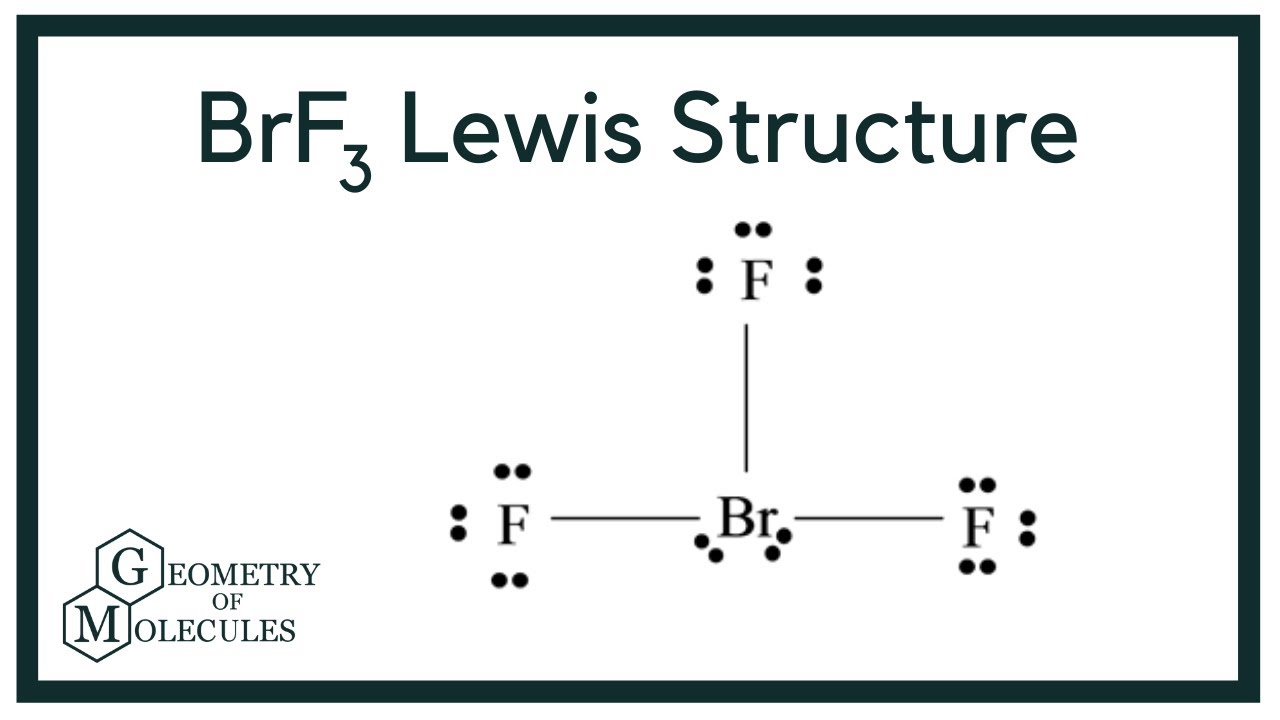
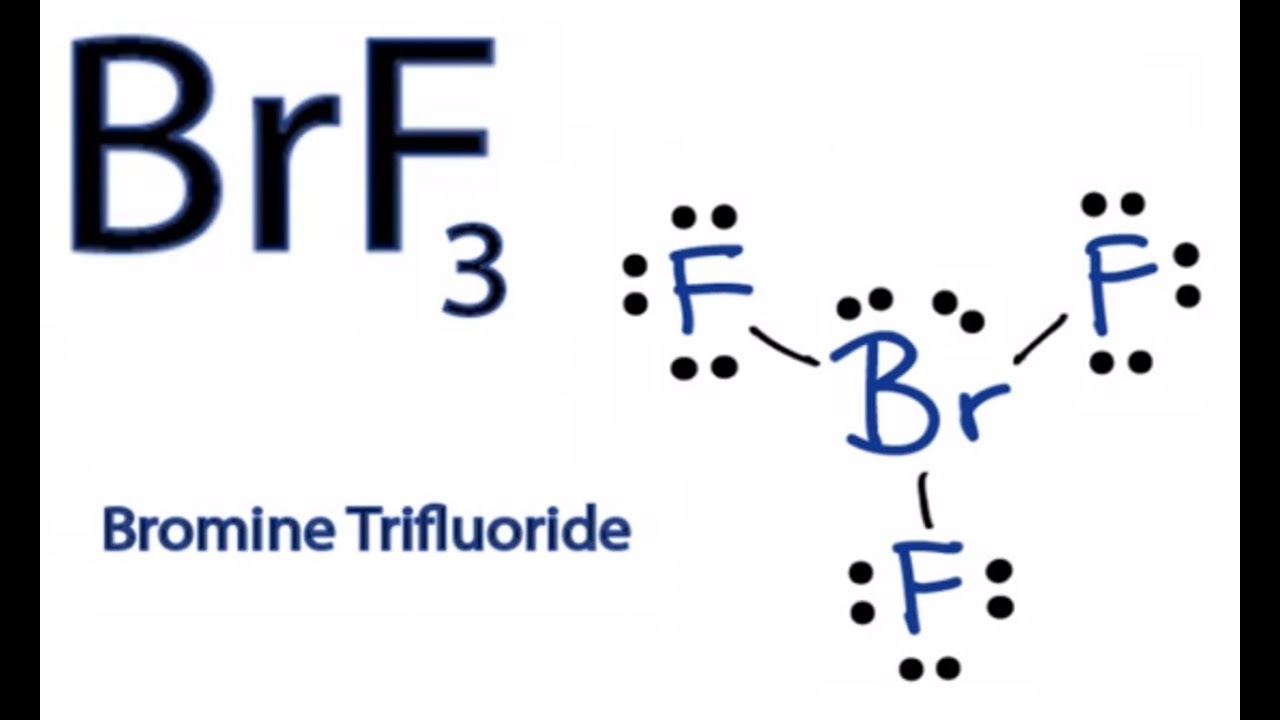
Draw The Lewis Structure For Brf3 - Web drawing the lewis structure for brf. Bromine and fluorine all each bring 7 valence. Here, the given molecule is brf3 (bromine trifluoride). Polar or nonpolar), draw the lewis structure for xef2. Web draw the lewis structure for brf3 in the window below and then answer the questions that follow. Try to draw the brf 3 lewis structure before watching the video. Try structures similar to bf 3 for more practice. Calculate the total number of valence electrons. #2 mark lone pairs on the atoms. The hybridization on the br is (sp, sp2, sp3, sp3d, sp3d2). Let’s discuss each step in more detail. 35k views 11 years ago chemistry lewis dot structures. How many valence electrons does a molecule of brf3 contain? Here, the given molecule is brf3 (bromine trifluoride). Web the hybridization on the br is (sp, sp2, sp3, sp3d, sp3d2). The brf3 lewis structure consists of one central atom, bromine (br), and three outer atoms, fluorine (f), at a bond angle of approximately 86.2°. In the brf 3 lewis structure bromine (br) is the least electronegative atom and goes in the center of the lewis structure. Brf3 draw the lewis structure of brf3 and then determine the ideal bonding angle. Web steps to form brf3 lewis structure. Bromine and fluorine all each bring 7 valence. Web drawing the lewis structure for brf. Brf3 does not follow the octet rule. The lewis structure for brf is similar to other structures like clf or f 2. Therefore, both of these elements will have a valency of 7. Web use these steps to correctly draw the brf 3 lewis structure: The cl atom is hybridized. How many electron groups are present around the central atom and what is the electron group geometry? A video explanation of how to draw the lewis dot structure for bromine trifluoride ,. First, determine the total number of valence electrons. Br and f are both halogens belonging to group 7 in the periodic table. How many nonbonding electron pairs are on the central atom? #3 calculate and mark formal charges on the atoms, if required. The hybridization on the xe is (sp, sp2, sp3, sp3d, sp3d2). Let’s discuss each step in more detail. Web the hybridization on the br is (sp, sp2, sp3, sp3d, sp3d2). The bromine atom (br) and each fluorine atom (f) are individually connected by a. Is brf3 polar or nonpolar? Draw the best lewis dot structure for brf3 in the correct molecular geometry [include formal charges, lone pair electrons and use dashed. The total number of valence electrons in brf3 = 7 + 7*3 You'll get a detailed solution from a subject matter expert that helps you learn core concepts. The brf3 lewis structure consists of one central atom, bromine (br), and three outer atoms, fluorine (f), at a bond angle of approximately 86.2°. The lewis structure for brf a 3 molecule. Web here is what is needed: 2.1k views 11 months ago. Draw the lewis structure for bromine trifluoride, brf3. #1 first draw a rough sketch. It is a powerful fluorinating agent. Drawing the lewis structure for brf3. What is the molecular geometry and ideal bond angles? Polar or nonpolar), draw the lewis structure for xef2. It is soluble in sulfuric acid but explodes on contact with water and organic compounds. Web the hybridization on the br is (sp, sp2, sp3, sp3d, sp3d2). Web here is what is needed: The molecule is (choose one: In order to draw the lewis structure of brf3, first of all you have to find the total number of valence electrons present in the brf3 molecule. Let’s discuss each step in more detail. A video explanation of how to draw the lewis dot structure for bromine trifluoride ,. Calculate the total number of valence electrons. Web draw the lewis structure for brf3 in the window below and then answer the questions that follow. The lewis structure for brf a 3 molecule can be drawn by identifying the electronic configuration and valen. In order to draw the lewis structure of brf3, first of all you have to find the total number of valence electrons present in the brf3 molecule. This problem has been solved! The brf3 lewis structure consists of one central atom, bromine (br), and three outer atoms, fluorine (f), at a bond angle of approximately 86.2°. Polar or nonpolar), draw the lewis structure for xef2. #3 calculate and mark formal charges on the atoms, if required. The hybridization on the xe is (sp, sp2, sp3, sp3d, sp3d2). Brf3 does not follow the octet rule. Using formal charges to determine how many bonds to make, a different perspective. Web bromine trifluoride is an interhalogen compound with the formula brf3 brf 3. The bromine atom (br) and each fluorine atom (f) are individually connected by a. Draw the lewis dot structure for the molecule brf3. Draw the best lewis dot structure for brf3 in the correct molecular geometry [include formal charges, lone pair electrons and use dashed and solid wedge bonds if necessary] 2. Drawing the lewis structure for brf3.
Formal charge on bromine atom of BrF3 molecule = (7 4(6/2)) =0
BrF3 Lewis Structure (Bromine Trifluoride) YouTube

draw the lewis structure for brf3 in the window below and then decide

Estructura de Brf3 Lewis, características 13 datos que debe conocer

How to draw BrF3 Lewis Structure? 3
How to Draw the Lewis Dot Structure for BrF3 Boron trifluoride YouTube

BrF3 Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram

Lewis Structure of BrF3 (bromine trifluoride) YouTube

BrF3 Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram

Brf3 Molecule
I Beleive It Should Look Like This:
The Hybridization On The Br Is (Sp, Sp2, Sp3, Sp3D, Sp3D2).
(Valence Electrons Are The Number Of Electrons Present In The Outermost Shell Of An Atom).
The Total Number Of Valence Electrons In Brf3 = 7 + 7*3
Related Post: