Draw The Lewis Structure For Sf4
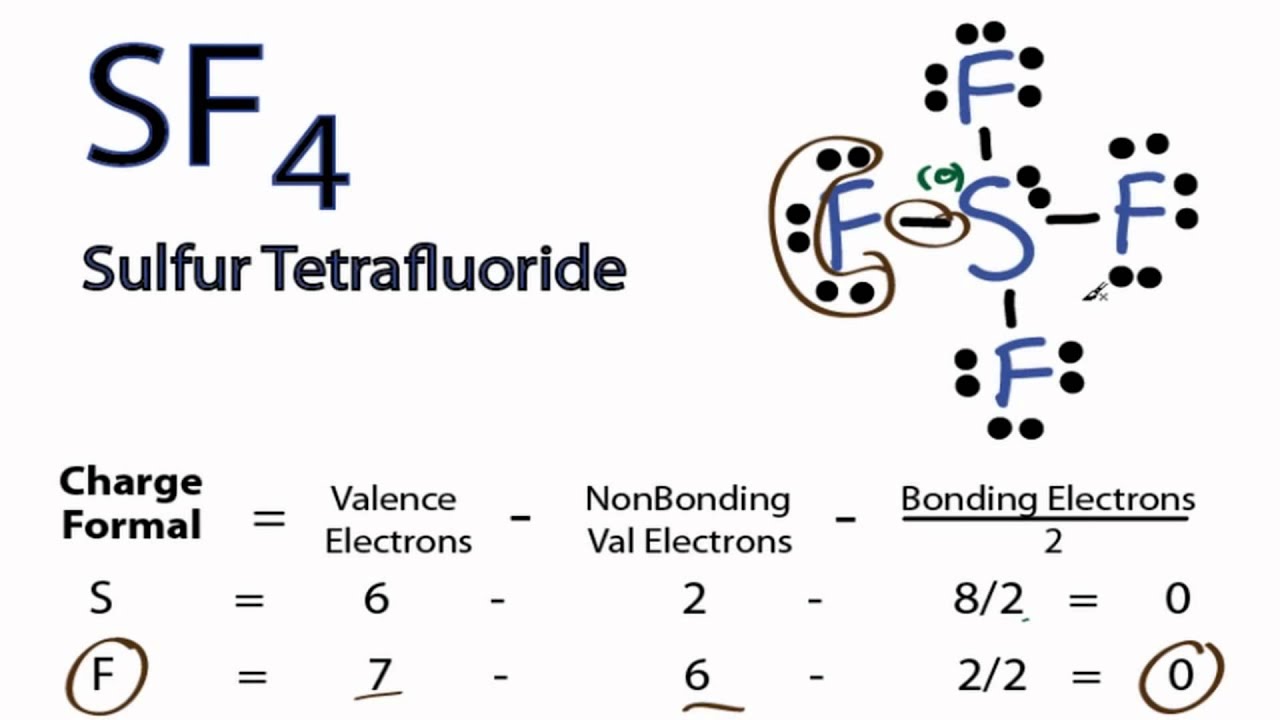
Draw The Lewis Structure For Sf4 - Web 5 steps to draw the lewis structure of sf4 step #1: Find the total valence electrons in sf4 molecule. Sf 4 is lewis structure with sulfur (s). Send feedback | visit wolfram|alpha. Remember that sulfur can hold more than 8 valence electrons. A lewis structure is a way to show how atoms share. Web learn the steps to draw the lewis structure of sf4 in just 1 minute.📌you can draw any lewis structures by following the simple steps mentioned in this artic. Web to draw the lewis structure for sf4, we start by determining the total number of valence electrons. The central atom in this compound is sulfur. In order to find the total valence electrons in sf4 molecule, first of all you. Check out this video for knowing the total. Find the total valence electrons in sf4 molecule. Web 178k views 10 years ago. #1 draw a rough skeleton structure. Web to draw a lewis structure we will first need to know the total number of valence electrons in sf4 which participate in the bond formation. Draw the lewis structure for sf4 in the window below and then answer the questions that follow. There are three lone pairs on each fluorine atom, and one lone. For the sf4 structure use the periodic table to find the. Web draw the lewis structure of sf4 showing all lone pairs. We draw lewis structures to predict: Here, the given molecule is sf4. Sulfur is in group 16 of the periodic table, so it has 6 valence electrons. Calculate the total number of valence electrons. A lewis structure is a way to show how atoms share. Web added jun 9, 2014 by webtester in chemistry. Here, the given molecule is sf4. Web lewis structure is a pictorial representation of the bonds and valence electrons in the molecule. Web to draw a lewis structure we will first need to know the total number of valence electrons in sf4 which participate in the bond formation. Remember that sulfur can hold more than 8 valence electrons. Web in. Find the total valence electrons in sf4 molecule. #1 draw a rough skeleton structure. #3 if needed, mention formal. I also go over formal charge, hybridization, shape and bond angle.**. Web here’s how you can easily draw the sf 4 lewis structure step by step: Web learn how to draw a lewis structure for sf4 and answer questions about the central sulfur atom's lone pairs, single bonds and double bonds. Web added jun 9, 2014 by webtester in chemistry. Web to draw a lewis structure we will first need to know the total number of valence electrons in sf4 which participate in the bond formation.. In this lewis structure of sf 4, center sulfur atom has made four single. We draw lewis structures to predict: Web learn the steps to draw the lewis structure of sf4 in just 1 minute.📌you can draw any lewis structures by following the simple steps mentioned in this artic. Web to draw a lewis structure we will first need to. Web the lewis structure of sf4 contains four single bonds, with sulfur in the center, and four fluorines on either side. #1 draw a rough skeleton structure. Remember that sulfur can hold more than 8 valence electrons. Web to draw a lewis structure we will first need to know the total number of valence electrons in sf4 which participate in. First, we arrange the atoms, then distribute the valence electrons, and finally, place them around the. For the sf4 structure use the periodic table to find the. Web lewis structure for sf4. Web 178k views 10 years ago. The central atom in this compound is sulfur. For the sf4 structure use the periodic table to find the. Web i quickly take you through how to draw the lewis structure of sf4, sulfur tetrafluoride. Web lewis structure for sf4. I also go over formal charge, hybridization, shape and bond angle.**. Identify the molecular geometry of sfa. Find the total valence electrons in sf4 molecule. What is the hybridization and formal charge on the sulfur? Draw the lewis structure for sf4. Web draw the lewis structure of sf4 showing all lone pairs. In this lewis structure of sf 4, center sulfur atom has made four single. Remember that sulfur can hold more than 8 valence electrons. #3 if needed, mention formal. First, we arrange the atoms, then distribute the valence electrons, and finally, place them around the. Web to draw lewis structures for molecules and polyatomic ions with one central atom. Web to draw a lewis structure we will first need to know the total number of valence electrons in sf4 which participate in the bond formation. Identify the molecular geometry of sfa. In this structure sulfur will have ten valence electrons. Web in this tutorial, we will learn how to draw the lewis structure of sf 4 step by step. Web to draw the structure of sf4 we will need to know the total number of valence electrons that exist in sf4. Web added jun 9, 2014 by webtester in chemistry. Web 178k views 10 years ago.
Lewis Structure Sof4
SF4 Molecular YouTube

SF4 Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram

SF4 Lewis Dot structureHow to draw the Lewis structure for SF4. neet

SF4 Lewis StructureLewis Structure for SF4 (Sulfur Tetrafluoride
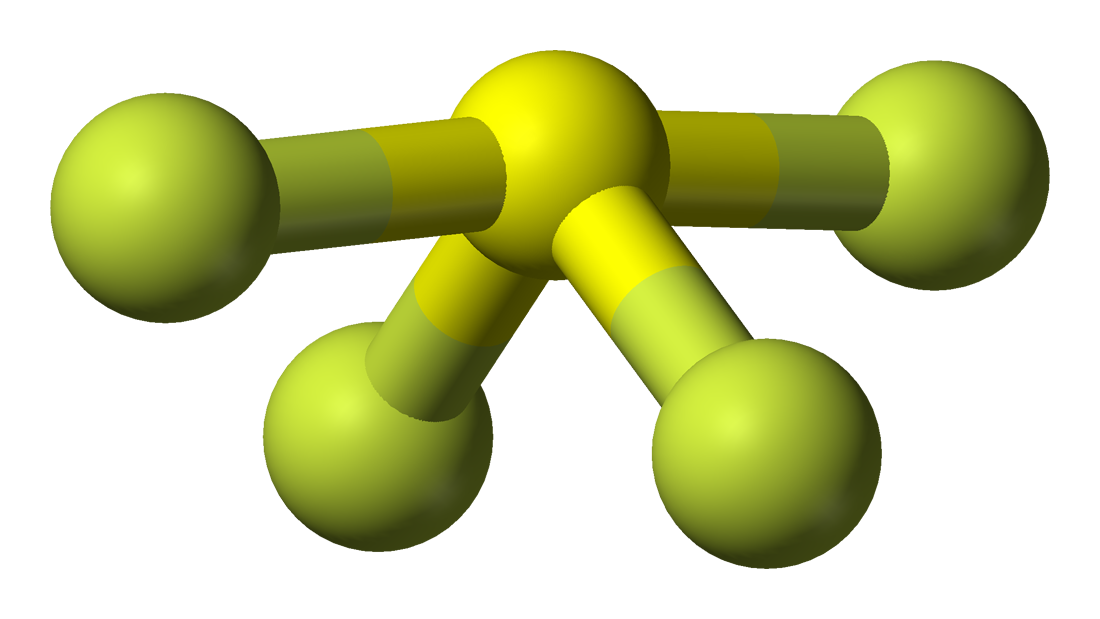
Geometría molecular del SF4, estructura de Lewis, ángulos de enlace y

SF4 Lewis Structure ,Valence Electrons,Formal Charge,Octet Rule

SF4 Lewis Structure How to Draw the Lewis Structure for SF4 YouTube

SF4 Lewis Structure How to Draw the Lewis Structure for SF4 YouTube

SF4 Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram
Check Out This Video For Knowing The Total.
Web Which Of The Following Compounds Is Sulfur Tetrafluoride?
Sulfur Is In Group 16 Of The Periodic Table, So It Has 6 Valence Electrons.
Web Here’s How You Can Easily Draw The Sf 4 Lewis Structure Step By Step:
Related Post: