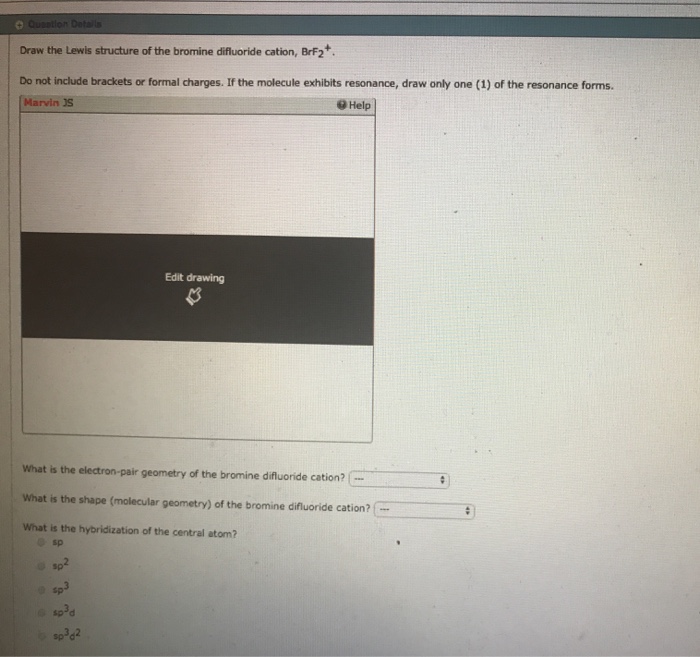
Draw The Lewis Structure For The Bromine Difluoride Ion
Draw The Lewis Structure For The Bromine Difluoride Ion - Calculate the total number of valence electrons. Web draw lewis structures for covalent compounds. (valence electrons are the number of electrons present in the outermost shell of an atom). The lewis structure for brf is similar to other structures like clf or f 2. Web chemistry questions and answers. Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. Please note that your structure can't be well described by a single lewis structure, because of extensive delocalization. Draw the lewis structure for the bromine difluoride ( brf, ) ion. Determine the total number of valence electrons in the molecule or ion. #1 draw a rough sketch of the structure. With brf lewis structure there are a total of 14 valence electrons. Web the hybridization of the atoms in this idealized lewis structure is given in the table below. (valence electrons are the number of electrons present in the outermost shell of an atom). How to draw the lewis structure for brf (bromine monofluoride) share. #2 mark lone pairs on. Draw the lewis structure of the bromine difluoride cation, brf2+ do not include brackets or formal charges. The lewis structure for brf is similar to other structures like clf or f 2. Hybridization in the best lewis structure. This is the lewis structure for brf2. When drawing the structure of an ion, be sure to add/subtract electrons to account for. Determine the total number of valence electrons in the molecule or ion. With brf lewis structure there are a total of 14 valence electrons. Web chemistry questions and answers. So we have an odd number of valence electrons in the lewis structure for brf2. Web in brf lewis structure, there is a single bond between the bromine and fluorine atom,. Determine the total number of valence electrons in the molecule or ion. #3 calculate and mark formal charges on the atoms, if required. Web draw lewis structures for ionic compounds. So we have an odd number of valence electrons in the lewis structure for brf2. Calculate the total number of valence electrons. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. #1 draw a rough sketch of the structure. Web draw lewis structures for covalent compounds. #1 first draw a rough sketch. Bromine has 7 valence electrons, as does fluorine. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. If the molecule exhibits resonance, draw only one (1) of the resonance forms. Web draw lewis structures for covalent compounds. This is the lewis structure for brf2. This widget gets the lewis structure of chemical compounds. This problem has been solved! #1 first draw a rough sketch. Draw the lewis structure of the bromine difluoride cation, brf2+ do not include brackets or formal charges. The astute reader may have noticed something: So, if you are ready to go with these 6 simple steps, then let’s dive right into it! The following procedure can be used to construct lewis electron structures for more complex molecules and ions. #1 first draw a rough sketch. Web chemistry questions and answers. See the big list of lewis structures. Web the following procedure can be used to draw lewis structure for simple molecules. That means one electron is. Web draw the lewis structure for the bromine difluoride (brf2) ion. With brf lewis structure there are a total of 14 valence electrons. #1 draw a rough sketch of the structure. Hybridization in the best lewis structure. Determine the total number of valence electrons in the molecule or ion. So, if you are ready to go with these 6 simple steps, then let’s dive right into it! The following procedure can be used to construct lewis electron structures for more complex molecules and ions. When drawing the structure of an ion, be sure to add/subtract electrons to. I’m super excited to teach you the lewis structure of brf in just 6 simple steps. #2 next, indicate lone pairs on the atoms. Web the hybridization of the atoms in this idealized lewis structure is given in the table below. So 7 plus 14 equals 21. How to draw the lewis structure for brf (bromine monofluoride) share. The following procedure can be used to construct lewis electron structures for more complex molecules and ions. Draw the lewis structure of the bromine difluoride cation, brf2+ do not include brackets or formal charges. Web draw the lewis structure for the bromine difluoride (brf2) ion. So we have an odd number of valence electrons in the lewis structure for brf2. #1 draw a rough sketch of the structure. Find more chemistry widgets in wolfram|alpha. With brf lewis structure there are a total of 14 valence electrons. So, if you are ready to go with these 6 simple steps, then let’s dive right into it! There is a single bond between the bromine atom (br) and each fluorine atom (f). Many of the ions that form have eight electrons in their valence shell. (valence electrons are the number of electrons present in the outermost shell of an atom).
BrF3 Lewis Structure (Bromine Trifluoride) YouTube

BrF5 Lewis Structure, Molecular Geometry, Hybridization, and Polarity
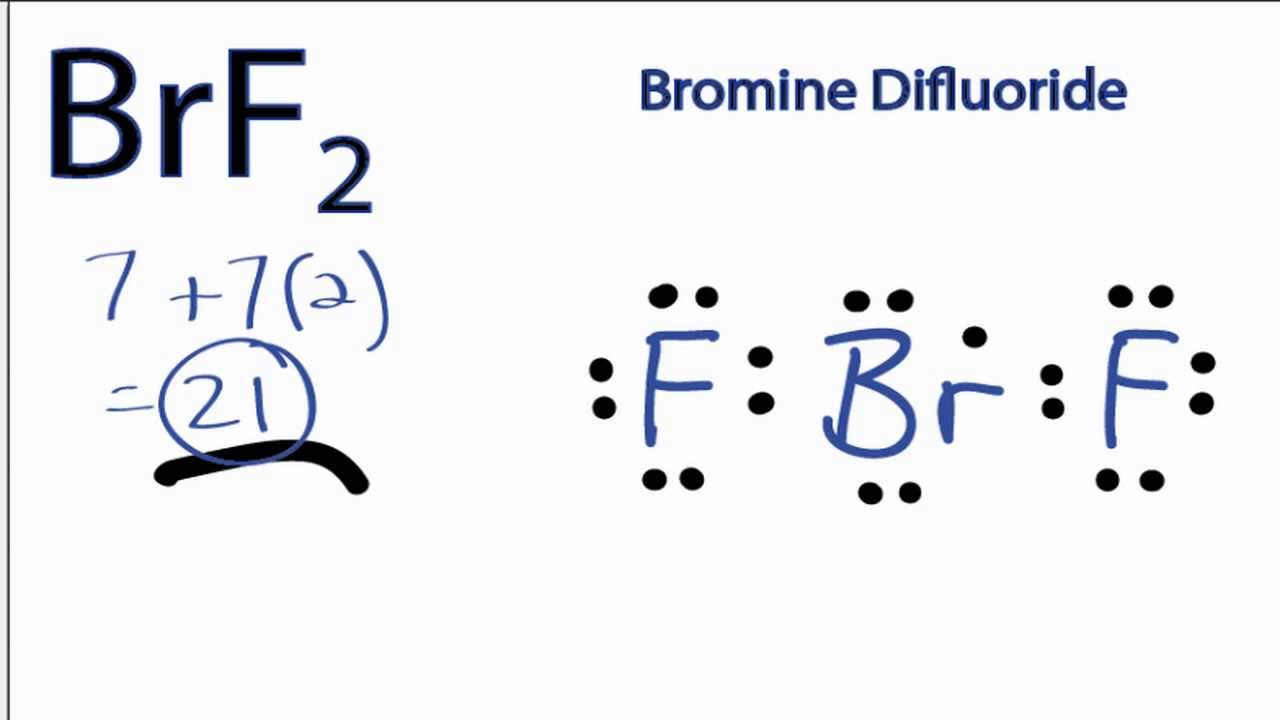
BrF2 Lewis Structure How to Draw the Lewis Structure for Bromine

BrF3 Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram

How to Draw the Lewis Dot Structure for BrF2 YouTube
Lewis structure Bromine pentafluoride Sulfur tetrafluoride Xenon
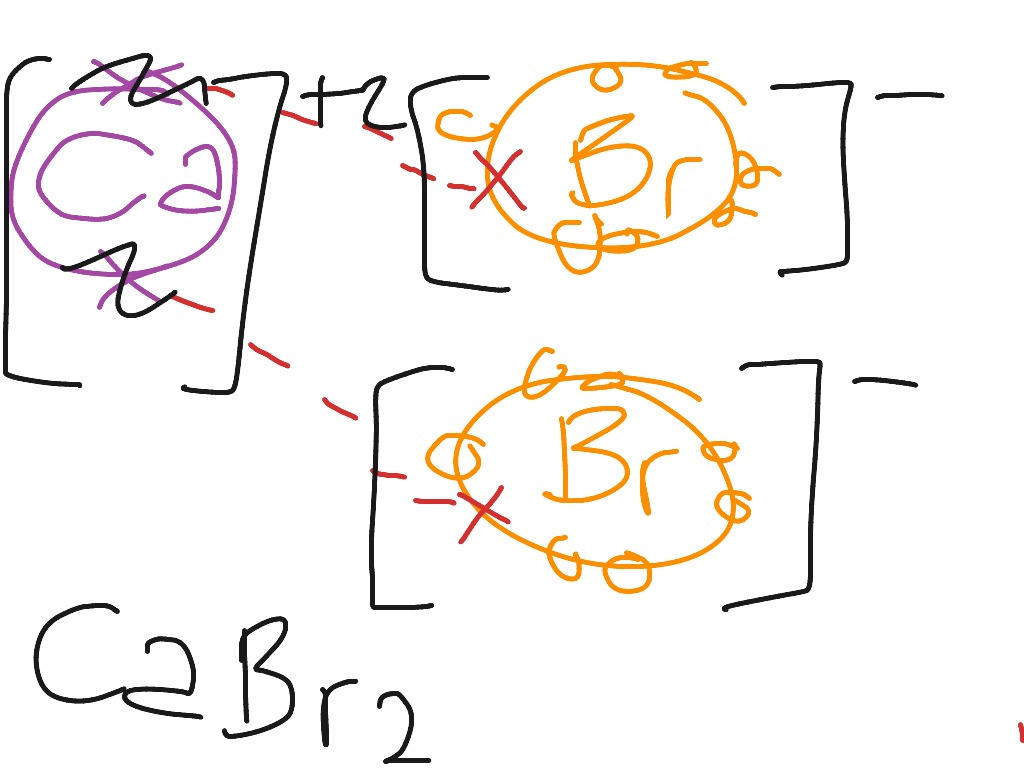
Lewis Structure For Bromine

Lewis Dot Diagram For Bromine

Brcl3 Lewis Structure
Solved Draw the Lewis structure of the bromine difluoride
Author:dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom.
Web The Following Procedure Can Be Used To Draw Lewis Structure For Simple Molecules.
A Lewis Diagram Shows How The Valence Electrons Are Distributed Around The Atoms In A Molecule.
This Problem Has Been Solved!
Related Post: