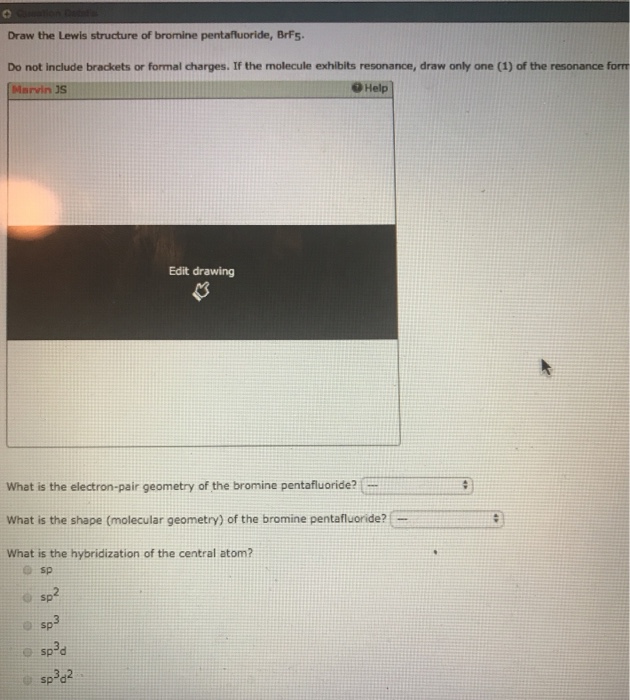
Draw The Lewis Structure For The Bromine Pentafluoride Molecule
Draw The Lewis Structure For The Bromine Pentafluoride Molecule - Images of the chemical structure of bromine pentafluoride are given below: You'll get a detailed solution from a subject matter expert that helps you learn core concepts. The central bromine molecule has an expanded valence. The valence electrons are the electrons in the outermost shell. The central atom in the lewis dot structure is bromine and it has 5 electron domains. Draw the lewis structure for the bromine pentafluoride (brf5) molecule. A chemical structure of a molecule includes the arrangement of atoms and the chemical bonds that hold the atoms together. This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web the lewis dot structure for bromine pentafluoride has a central bromine atom surrounded by five fluorine atoms. View the full answer step 2. Web draw the lewis structure by representing each bond as a line and each lone pair as two dots. The bromine atom has 1 lone pair while all the five fluorine atoms have 3 lone pairs. By the end of this section, you will be able to: (ax 5 e 1 ). The bromine pentafluoride molecule contains a total of 5 bond (s). Web the lewis dot structure for bromine pentafluoride has a central bromine atom surrounded by five fluorine atoms. The central atom in the lewis dot structure is bromine and it has 5 electron domains. Web < 18 19 20 21 22 23 24 25 draw the lewis structure for. Web welcome back to our channel and today we are going to share yet another video for determining the lewis structure of the given molecule over here: This structure is also available as a 2d mol file or as a computed 3d sd file the 3d structure may be viewed using java or. Web from the lewis dot structure of. There are no lone pairs on. View the full answer step 2. The valence electrons are the electrons in the outermost shell. An explanation of the molecular geometry for the brf5 (bromine pentafluoride) including a description of the brf5 bond angles. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. By the end of this section, you will be able to: Count the total valence electrons: This problem has been solved! It can be observed from the brf5 lewis structure that there are five fluorine atoms surrounding the central bromine atom. Web welcome back to our channel and today we are going to share yet another video for determining the. This structure is also available as a 2d mol file or as a computed 3d sd file the 3d structure may be viewed using java or. Web he bromine pentafluoride (brf5) molecule.draw the lewis structure for the bromine pentafluoride (brf5) molecule. Web chemistry questions and answers. Web there are 2 steps to solve this one. Web welcome back to our. This problem has been solved! By the end of this section, you will be able to: The bromine pentafluoride molecule contains a total of 5 bond (s). (ax 5 e 1 ). Send feedback | visit wolfram|alpha. For the brf5 structure use the periodic table to find the total number of valence electrons. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. This widget gets the lewis structure of chemical compounds. Added jun 9, 2014 by webtester in chemistry. The bromine pentafluoride molecule contains a total of 5 bond (s). For the brf5 structure use the periodic table to find the total number of valence electrons. Web lewis structure of brf5 contains five single bonds between the bromine (br) atom and each fluorine (f) atom. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Drawing the lewis structure of bromine pentafluoride (brf5): The. An explanation of the molecular geometry for the brf5 (bromine pentafluoride) including a description of the brf5 bond angles. This widget gets the lewis structure of chemical compounds. Draw the lewis structure of bromine pentafluoride, brfs do not include brackets or formal charges. Web bromine pentafluoride comprises 5 fluorine atoms, all pulled together by the central bromine atom. You'll get. An explanation of the molecular geometry for the brf5 (bromine pentafluoride) including a description of the brf5 bond angles. Draw lewis structures depicting the bonding in simple molecules. Lewis structures show all of the valence electrons in an atom or molecule. This structure is also available as a 2d mol file or as a computed 3d sd file the 3d structure may be viewed using java or. It can be observed from the brf5 lewis structure that there are five fluorine atoms surrounding the central bromine atom. Find the total valence electrons in brf5 molecule. The hybridization of the central atom is sp3d2. This problem has been solved! The difference between both the values is 1.02 which is greater than 0.4 so the brf5 molecule is a polar molecule. This problem has been solved! 37k views 3 years ago. This widget gets the lewis structure of chemical compounds. The electron domain geometry is trigonal bipyramidal. There are no lone pairs on. The structure should show the central bromine atom bonded to five fluorine atoms, with each fluorine atom having three lone pairs. Web there are 2 steps to solve this one.
BrF5 Lewis Dot Structure (Bromine Pentafluoride) YouTube

How to Draw the Lewis Dot Structure for BrF5 Bromine pentafluoride

Draw the Lewis structure of bromine pentafluoride, Br… SolvedLib

BrF5 Molecular Geometry & Bond Angles (Bromine Pentafluoride) YouTube
Solved Draw the Lewis structure of bromine pentafluoride,
Bromine Pentafluoride Lewis Structure Molecular Geometry Molecule PNG

Bromine pentafluoride Wikiwand
What is the Hybridization of Bromine Pentafluoride

AP08.10 Lewis Dot structure bromine pentafluoride BrF5 YouTube
Molecular Geometry CK12 Foundation
Send Feedback | Visit Wolfram|Alpha.
The Valence Electrons Are The Electrons In The Outermost Shell.
Draw The Lewis Structure For The Bromine Pentafluoride (Brf5) Molecule.
You'll Get A Detailed Solution From A Subject Matter Expert That Helps You Learn Core Concepts.
Related Post: