Draw The Lewis Structure For The Phosphorus Pentabromide Molecule
Draw The Lewis Structure For The Phosphorus Pentabromide Molecule - Here, the given molecule is pbr5 (phosphorus pentabromide). Lewis structures show all of the valence electrons in an atom or molecule. Web phosphorus pentabromide is a reactive, yellow solid of formula p br 5, which has the structure [pbr4]+ br − (tetrabromophosphonium bromide) in the solid state but in the vapor phase is completely dissociated to pbr3 and br2. Added jun 9, 2014 by webtester in chemistry. You'll get a detailed solution from a subject matter expert. In order to draw the lewis structure of pbr5, first of all you have to find the total number of valence. How to draw pbr4+ lewis structure? #2 mention lone pairs on the atoms. The compound has one molecule of phosphorus and five bromine molecules. This problem has been solved! In order to draw the lewis structure of pbr5, first of all you have to find the total number of valence. The compound has one molecule of phosphorus and five bromine molecules. #1 draw a rough skeleton structure. In this case, there is no central atom, so we distribute the electrons around both atoms. You start by placing the phosphorus. To use the vsepr model to predict molecular geometries. It is a chemical formula for phosphorus pentabromide.to understand the lewis. The compound has one molecule of phosphorus and five bromine molecules. Web it is corrosive in nature. A lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. Also tells us about the electrons that are of nonbonding type. In the lewis structure for pbr 5 there are a total of 40 valence electrons. Web phosphorus pentabromide is a reactive, yellow solid of formula p br 5, which has the structure [pbr4]+ br − (tetrabromophosphonium bromide) in the solid state but in the vapor phase is completely dissociated. Send feedback | visit wolfram|alpha. Web how to draw lewis structure for pbr5. There are 5 single bonds between the phosphorus atom (p) and each bromine atom (br). In the lewis structure for pbr 5 there are a total of 40 valence electrons. Web pbr5 molecular geometry, lewis structure, shape, bond angle, and more. The valence electrons are the electrons in the outermost shell. Web draw a skeleton structure of the molecule. Here, the given molecule is pbr5 (phosphorus pentabromide). How to draw pbr4+ lewis structure? To use the vsepr model to predict molecular geometries. Web draw a skeleton structure of the molecule. Web how to draw lewis structure for pbr5. Web the pentahalides of phosphorus are lewis acids because of the empty valence d orbitals of phosphorus. A lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. A lewis structure is a way to show how atoms share. Calculate the total number of valence electrons. Web in the pbr 5 lewis structure phosphorus (p) is the least electronegative so it goes in the center. Computed by pubchem 2.2 (pubchem release 2021.10.14) dates. Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. Phosphorus pentabromide written as pbr5 in the chemistry equations is a. Web the lewis structure for phosphorus pentabromide (pbr5) can be determined by following the octet rule. Web it is corrosive in nature. This problem has been solved! In the lewis structure for pbr 5 there are a total of 40 valence electrons. How many electrons are involved and are bonding electrons. This problem has been solved! Web hey guys,in this video, we are going to learn about the lewis structure of pbr5. It appears to be a yellow crystalline solid. #3 if needed, mention formal charges on the atoms. There are 5 single bonds between the phosphorus atom (p) and each bromine atom (br). Draw the lewis structure for the phosphorus pentabromide (pbrz) molecule. Web the pentahalides of phosphorus are lewis acids because of the empty valence d orbitals of phosphorus. Web it is corrosive in nature. In this case, there is no central atom, so we distribute the electrons around both atoms. Web hey guys,in this video, we are going to learn about. A lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. Web it is corrosive in nature. Phosphorus has 5 valence electrons, while bromine has 7 valence electrons each. These compounds readily react with halide ions (lewis bases) to give the anion px 6 −. By using lewis concept of bonding we can clearly understand about how the bonding occurs in a molecule. There are 5 single bonds between the phosphorus atom (p) and each bromine atom (br). Web to draw lewis structures for molecules and polyatomic ions with one central atom. Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. Here, the given molecule is pbr5 (phosphorus pentabromide). Web draw a skeleton structure of the molecule. Added jun 9, 2014 by webtester in chemistry. Also tells us about the electrons that are of nonbonding type. Lewis structure representation can be used to calculate the formal charge of atoms present in a molecule. Five pairs will be used in the chemical bonds between the p and br. Let us see the steps in drawing lewis structure of pbr4+. A lewis structure is a way to show how atoms share electrons when they form a molecule.
Phosphorus pentabromide Nitrogen tribromide Phosphorus tribromide

In this video we are going to learn about the Lewis structure of PBr5

Phosphorus Lewis Dot Diagram

How to Write the Formula for Phosphorus pentabromide YouTube

Phosphorus Pentabromide Lewis Structure Liqurus

PBr5 Lewis StructureLewis Structure of PBr5 (Phosphorus Pentabromide

Phosphorus pentabromide Phosphorus tribromide Lewis structure Crystal
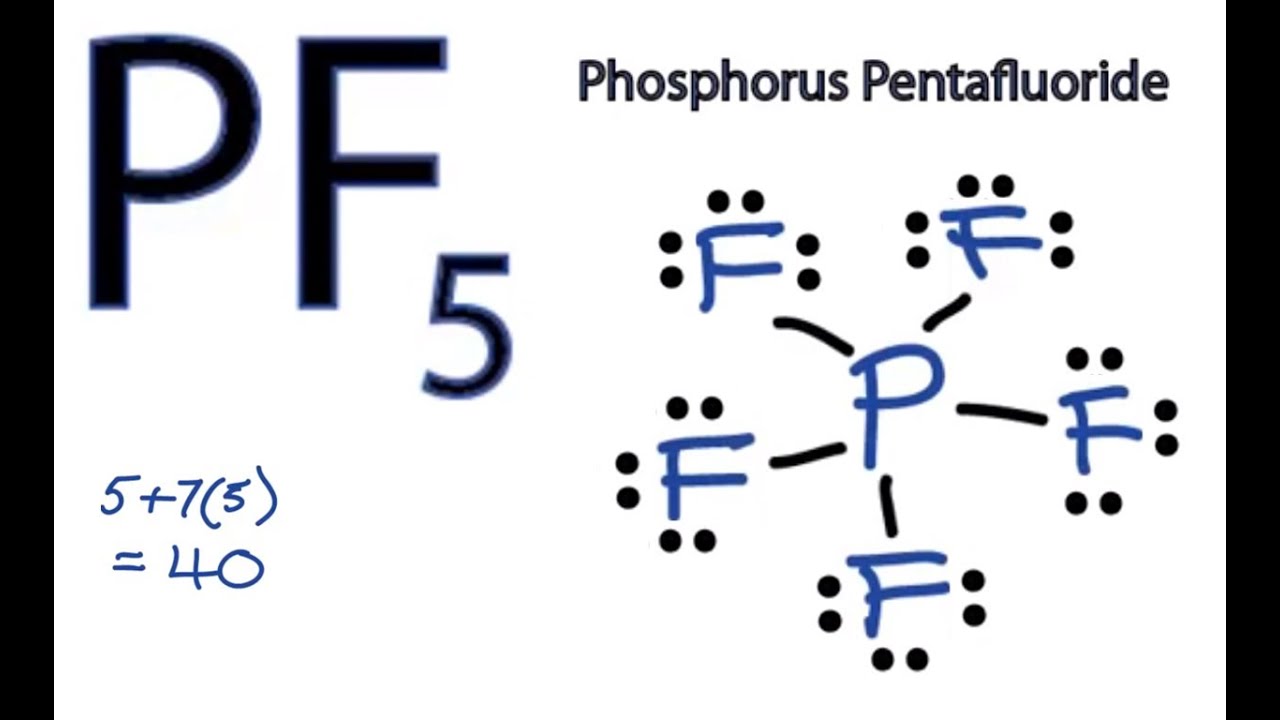
What Is The Formal Charge On Phosphorus In A Lewis Structure Drawing Easy
PPT Lewis Structures and Molecular Geometry PowerPoint Presentation
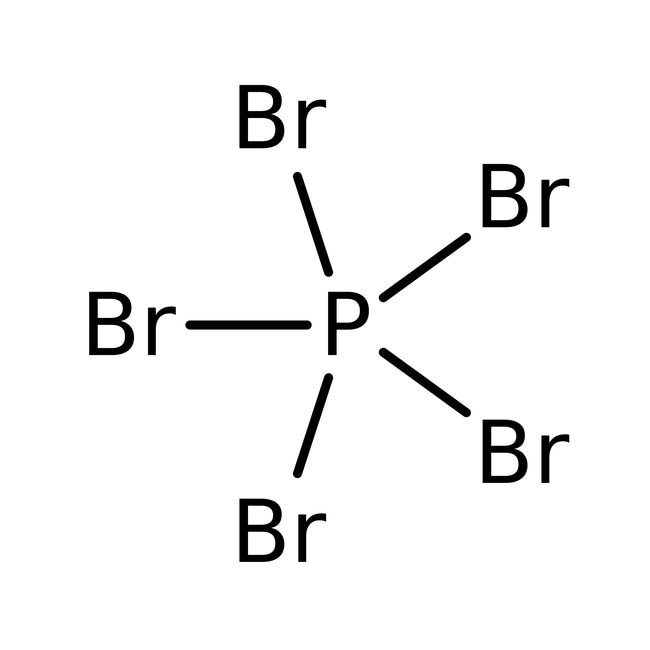
Phosphorus pentabromide, 95, Thermo Scientific™
Distribute The Remaining Electrons As Lone Pairs On The Terminal Atoms.
This Widget Gets The Lewis Structure Of Chemical Compounds.
How To Draw Pbr4+ Lewis Structure?
Draw The Lewis Structure For The Phosphorus Pentabromide (Pbrz) Molecule.
Related Post: