Draw The Lewis Structure Of Co3 2
Draw The Lewis Structure Of Co3 2 - Web put one electron pair in each bond 4. Sometimes one lewis structure is not enough. Each of the singly bonded oxygen atoms bears a formal charge of ‐1 and all other atoms are. This widget gets the lewis structure of chemical compounds. B) determine the electronegativity difference (δen) of the co bond (electronegativities: Draw the lewis dot structure of the following. It consists of one carbon atom (c) bonded to three oxygen atoms (o) through double bonds, with one of the oxygen atoms also carrying a negative charge. The answer is 3 may i recommend a video () let’s consider the lewis structure of the carbonate ion, co32‐. Count the total number of valence electrons. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. The centre one) has a full octet. The valence electrons are the electrons in the. Location of carbon and oxygen on the periodic table | image: This widget gets the lewis structure of chemical compounds. Move electrons so all atoms (esp. Draw the lewis dot structure of the following. 174k views 3 years ago. 31k views 1 year ago. Sometimes one lewis structure is not enough. The answer is 3 may i recommend a video () let’s consider the lewis structure of the carbonate ion, co32‐. It consists of one carbon atom (c) bonded to three oxygen atoms (o) through double bonds, with one of the oxygen atoms also carrying a negative charge. The valence electrons are the electrons in the. Web lewis structure of carbonate ion is drawn in this tutorial step by step. Sometimes one lewis structure is not enough. 321k views 12 years. Web to draw lewis structures for molecules and polyatomic ions with one central atom. Determine the total number of valence electrons in the molecule by adding up the valence electrons of each atom. Location of carbon and oxygen on the periodic table | image: The answer is 3 may i recommend a video () let’s consider the lewis structure of. 174k views 3 years ago. Web to draw lewis structures for molecules and polyatomic ions with one central atom. The lewis structure for co2− 3 is shown in the figure. Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. Sometimes one lewis structure is not enough. You will learn about these facts in this tutorial. A lewis structure is a way to show how atoms share electrons when they form a molecule. Web added jun 9, 2014 by webtester in chemistry. Draw the lewis dot structure of the following. The centre one) has a full octet. It consists of one carbon atom (c) bonded to three oxygen atoms (o) through double bonds, with one of the oxygen atoms also carrying a negative charge. Sometimes one lewis structure is not enough. The correct lewis structure for this ion has one carbon‐oxygen double bond, and two carbon‐oxygen single bonds. Draw the lewis structure for co2− 3. Some molecules. Determine the total number of valence electrons in the molecule by adding up the valence electrons of each atom. The centre one) has a full octet. Each of the singly bonded oxygen atoms bears a formal charge of ‐1 and all other atoms are. This can be calculated by multiplying the valence electrons of each atom. Count the total number. B) determine the electronegativity difference (δen) of the co bond (electronegativities: Draw the lewis structure for co2− 3. 321k views 12 years ago every video. Some molecules or ions cannot be adequately described by a single lewis structure. You will learn about these facts in this tutorial. B) determine the electronegativity difference (δen) of the co bond (electronegativities: Each of the singly bonded oxygen atoms bears a formal charge of ‐1 and all other atoms are. Some molecules or ions cannot be adequately described by a single lewis structure. Lewis structures show all of the valence electrons in an atom or molecule. Web added jun 9, 2014. 321k views 12 years ago every video. The valence electrons are the electrons in the. Some molecules or ions cannot be adequately described by a single lewis structure. Valence electrons are the electrons in the outermost shell of an atom that participate in chemical bonding. Move electrons so all atoms (esp. Therefore it is put in the center of the dot structure. Send feedback | visit wolfram|alpha. 31k views 1 year ago. The lewis structure for co2− 3 is shown in the figure. The correct lewis structure for this ion has one carbon‐oxygen double bond, and two carbon‐oxygen single bonds. 3.5) c) determine the polarity of the co bond. Location of carbon and oxygen on the periodic table | image: The answer is 3 may i recommend a video () let’s consider the lewis structure of the carbonate ion, co32‐. Web lewis structure of carbonate ion is drawn in this tutorial step by step. Determine the total number of valence electrons in the molecule by adding up the valence electrons of each atom. A lewis structure is a way to show how atoms share electrons when they form a molecule.
Draw Lewis dot structure for CO3 2( carbonate ion) YouTube

Lewis structure of CO3 2 ion YouTube

CO32 Lewis Structure How to Draw the Lewis Structure for CO3 2
[Solved] 8. Draw the Lewis structures for CO2 and CO, and predict the
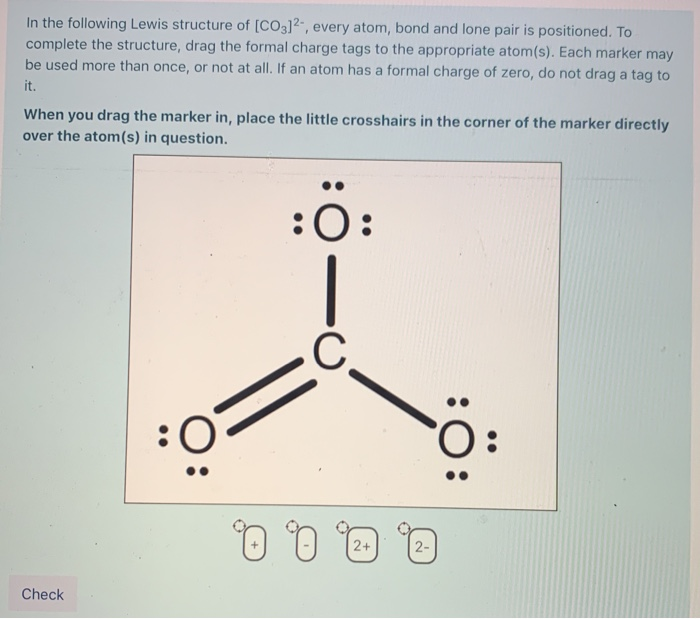
Solved In the following Lewis structure of [CO3)2, every

23.Lewis Dot Structure of CO3 2 How to Draw Lewis StructuresClass
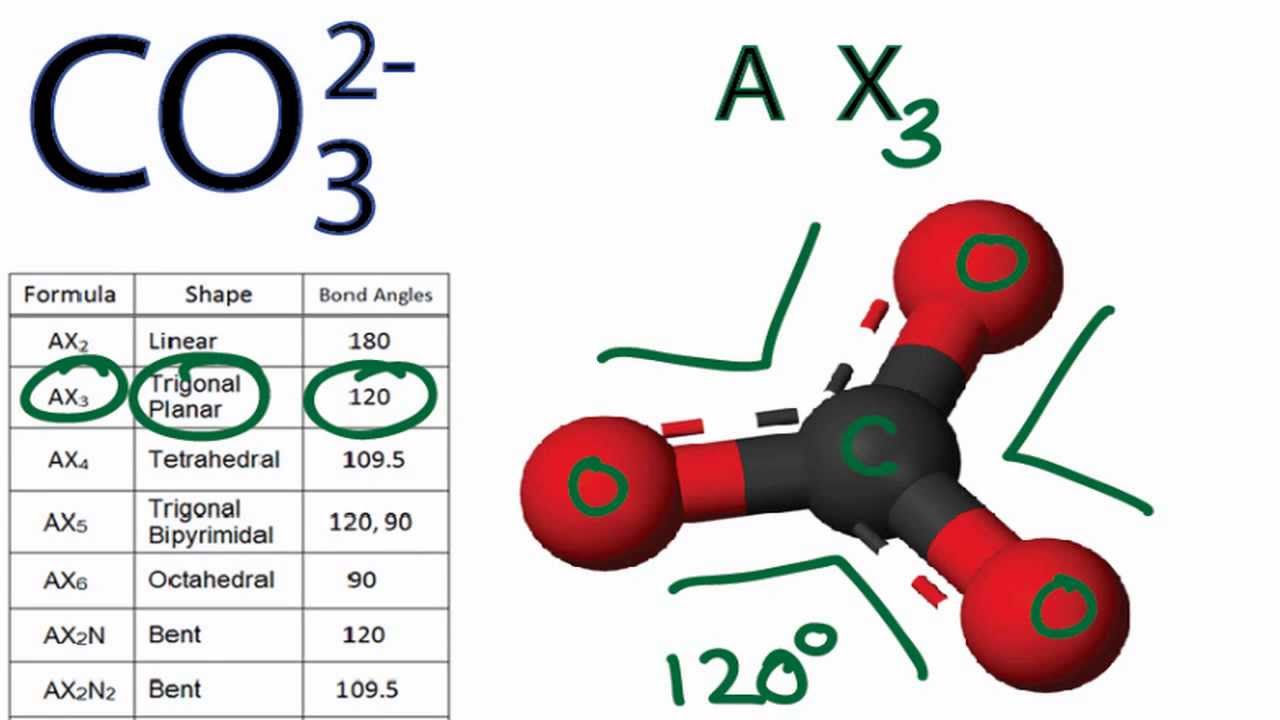
Hướng dẫn vẽ co3 2 lewis structure đầy đủ và chi tiết nhất

Lewis Structure for CO3 2 (Carbonate ion) YouTube

How To Draw The Lewis Structure of CO3 2 (Carbonate Ion) Chemistry

How to draw the Lewis structure of CO3 2 (Carbonate ion) YouTube
Co2− 3, H Clo4, H N O3.
Fill Outer Atoms (O) With Electrons First.
Calculate The Total Number Of Valence Electrons.
Web Put One Electron Pair In Each Bond 4.
Related Post: