Fda Pdufa Calendar
Fda Pdufa Calendar - Web the fda announced the reauthorization of the prescription drug user fee act (pdufa) for fiscal years (fys) 2023 through 2027, which provides the agency with. The pdufa date is 10 months after the drug application has. Web the food and drug administration (fda or agency) is announcing a virtual public meeting to discuss proposed recommendations for the reauthorization of the prescription drug. Web the prescription drug user fee act (pdufa) date marks the deadline by which the fda must complete its review of drug or device applications. Compiling pdufa dates is hard. Web track upcoming pdufa drug approval dates and fda advisory committee meetings with fda tracker. Web june 1, 2023 12:17 am utc. The prescription drug user fee act (pdufa) date refers to the deadline set by the us food and drug administration (fda) for reviewing a new drug application. By establishing timelines and goals, it provides. Web the pdufa calendar plays a vital role in expediting fda drug reviews and shaping the pharmaceutical landscape. Under pdufa, cder reviews new drug and biologics applications targeting. Compiling pdufa dates is hard. That’s double the number of. The pdufa date is 10 months after the drug application has. The prescription drug user fee act (pdufa) date refers to the deadline set by the us food and drug administration (fda) for reviewing a new drug. Compiling pdufa dates is hard. The pdufa date is 10 months after the drug application has. In united states pharmaceutical regulatory practice, the pdufa date is the. The current legislative authority for pdufa (pdufa v),. Web find future pdufa dates and advisory committee meetings for biotech companies on this calendar. The prescription drug user fee act (pdufa) date refers to the deadline set by the us food and drug administration (fda) for reviewing a new drug application. By joseph lee · january 21, 2015. Web the fda’s 2024 pdufa calendar is far from complete, but already it contains several dates worth watching, from the first new drug class for schizophrenia. Under pdufa, cder reviews new drug and biologics applications targeting. Web the prescription drug user fee act (pdufa) date marks the deadline by which the fda must complete its review of drug or device applications. By joseph lee · january 21, 2015. Web the pdufa calendar plays a vital role in expediting fda drug reviews and shaping the pharmaceutical landscape.. Sign up or log in to access the enhanced fda calendar with more data. The current legislative authority for pdufa (pdufa v),. Compiling pdufa dates is hard. Web here's a look at important regulatory action dates to watch. Web the fda announced the reauthorization of the prescription drug user fee act (pdufa) for fiscal years (fys) 2023 through 2027, which. Web what is a pdufa date? Early alzheimer’s disease pdufa date: Compiling pdufa dates is hard. Web the federal food, drug, and cosmetic act (the fd&c act), as amended by the prescription drug user fee act of 2017 (pdufa vi), authorizes fda to assess and collect fees for. Fda has set targets dates for decisions on at least 15 therapeutics. Early alzheimer’s disease pdufa date: Web fda has a busy pdufa calendar next month, with goal dates coming up for at least 13 applications, 10 of which are for new therapies. Web at least 18 pdufa dates on fda’s calendar for may. Once the fda accepts a filing for the approval of a drug, the agency must complete its review. Sign up or log in to access the enhanced fda calendar with more data. Web fda’s center for drug evaluation and research january 2023. Web the federal food, drug, and cosmetic act (the fd&c act), as amended by the prescription drug user fee act of 2017 (pdufa vi), authorizes fda to assess and collect fees for. Fda periodically conducts meetings. Web find future pdufa dates and advisory committee meetings for biotech companies on this calendar. Web fda’s center for drug evaluation and research january 2023. The pdufa date is 10 months after the drug application has. Web the fda’s 2024 pdufa calendar is far from complete, but already it contains several dates worth watching, from the first new drug class. Web fda’s center for drug evaluation and research january 2023. Compiling pdufa dates is hard. Once the fda accepts a filing for the approval of a drug, the agency must complete its review process within 10 months in most cases. Web the original pdufa date of march 16 was extended by three months, as the fda needed additional time to. Early alzheimer’s disease pdufa date: The decisions include sarepta’s dmd candidate, which could become the first gene therapy to receive. Web june 1, 2023 12:17 am utc. By establishing timelines and goals, it provides. Web the fda’s 2024 pdufa calendar is far from complete, but already it contains several dates worth watching, from the first new drug class for schizophrenia in. Web learn about the prescription drug user fee act (pdufa), a program that allows fda to collect fees from companies that submit certain human drug and biologic applications for. That’s double the number of. Web the federal food, drug, and cosmetic act (the fd&c act), as amended by the prescription drug user fee act of 2017 (pdufa vi), authorizes fda to assess and collect fees for. Web find future pdufa dates and advisory committee meetings for biotech companies on this calendar. Compiling pdufa dates is hard. Once the fda accepts a filing for the approval of a drug, the agency must complete its review process within 10 months in most cases. Web the pdufa decision date is set for jan. Web the federal food, drug, and cosmetic act (the fd&c act), as amended by the prescription drug user fee act of 2017 (pdufa vi), authorizes fda to assess and collect fees for. Web the fda announced the reauthorization of the prescription drug user fee act (pdufa) for fiscal years (fys) 2023 through 2027, which provides the agency with. The current legislative authority for pdufa (pdufa v),. The prescription drug user fee act (pdufa) date refers to the deadline set by the us food and drug administration (fda) for reviewing a new drug.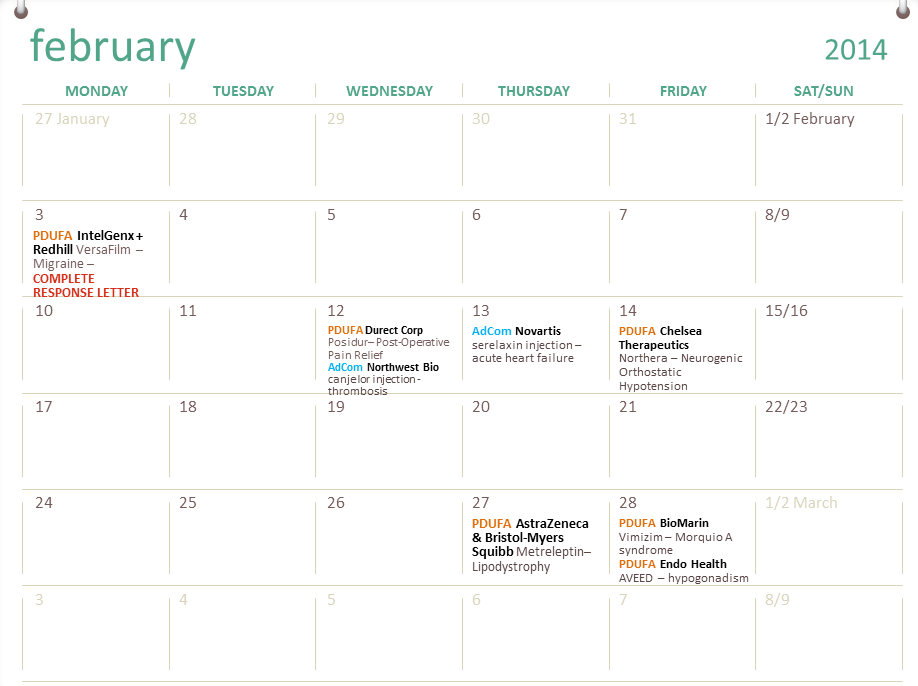
FDA Drug Calendar PDUFAs, Drug Approvals and Rejections
PDUFA Calendar Bio Data Studio

FDA PDUFA Calendar, PDUFA Dates, FDA Approval Dates BiopharmIQ
Fda Pdufa Calendar Customize and Print
![]()
Fda Pdufa Calendar Customize and Print
Fda Pdufa Calendar Customize and Print

PDUFA Dates, FDA Approval Dates BiopharmIQ
![]()
FDA Calendar FDA Tracker

Fda Pdufa Calendar Customize and Print
![]()
FDA Calendar FDA Tracker
Web Track Upcoming Pdufa Drug Approval Dates And Fda Advisory Committee Meetings With Fda Tracker.
Web The Most Comprehensive Fda Pdufa Date Calendar.
Web Fda Has A Busy Pdufa Calendar Next Month, With Goal Dates Coming Up For At Least 13 Applications, 10 Of Which Are For New Therapies.
Web At Least 18 Pdufa Dates On Fda’s Calendar For May.
Related Post: