How Do You Draw An Electron Dot Diagram
How Do You Draw An Electron Dot Diagram - Draw a lewis electron dot symbol for a given atom. Web you can use a drawing or use small beads or objects to represent electrons and work out the electron arrangement. Lewis electron dot diagrams for ions have fewer (for cations) or more (for anions) dots than the corresponding atom. Web how to draw electron dot structures? A beryllium atom, with two valence electrons, would have the electron dot diagram below. The number of dots equals the number of valence electrons in the atom. The diagram is also called a lewis dot diagram, lewis dot formula, or electron dot diagram. Draw a lewis electron dot diagram for an atom or a monatomic ion. More complicated versions can be used to show the bond between different atoms in a molecule. Web a lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or a lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. Give examples for molecules and ions that do not follow the octet rule. Lewis electron dot diagrams use dots to represent valence electrons around an atomic symbol. Web these diagrams are helpful because they allow us to show how atoms are connected, and when coupled with valence shell electron repulsion theory (vsepr), we can use lewis structures to predict the. Web a lewis structure is a diagram that shows the chemical bonds between atoms in a molecule and the valence electrons or lone pairs of electrons. Then we place the valence electrons around the sides of the box with each side representing an orbital in the outermost energy level. The number of dots equals the number of valence electrons in. How many valence electrons does magnesium have? The diagram is also called a lewis dot diagram, lewis dot formula, or electron dot diagram. Web these diagrams are used as a shorthand notation to show the number of valence electrons in an atom. Web here's some of the guidelines for drawing dot structures. This is sometimes called the bohr, or the. The diagram is also called a lewis dot diagram, lewis dot formula, or electron dot diagram. Here we have changed the electrons on the hydrogen atoms to be dots. Web draw the completed dot and cross diagram. How many valence electrons does magnesium have? In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. Draw a lewis electron dot diagram for an atom or a monatomic ion. Since electrons repel each other, the dots for a given atom are distributed evenly around the symbol before they are paired. Give examples for molecules and ions that do not follow the octet rule. Web to draw the lewis electron dot diagram we picture in our minds. Web to draw the lewis electron dot diagram we picture in our minds the symbol for mg in a box with all of its core electrons (i.e., 1 s2 2 s2 2 p6 ). How many valence electrons does magnesium have? A lewis electron dot formula comprises one dot for every valence electron and an element’s symbol. Draw a lewis. Web electron dot diagrams are diagrams in which the valence electrons of an atom are shown as dots distributed around the element's symbol. More complicated versions can be used to show the bond between different atoms in a molecule. The number of dots equals the number of valence electrons in the atom. Web to draw the lewis electron dot diagram. Web you can use a drawing or use small beads or objects to represent electrons and work out the electron arrangement. Web electron dot diagrams are diagrams in which the valence electrons of an atom are shown as dots distributed around the element's symbol. Web lewis dot structures, or lewis structures for short, are visuals that represent the outermost shell. Assess the stability of a structure by considering formal charges of atoms. Draw resonance structures of some molecules. In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. Atoms and the periodic table. This is sometimes called the bohr, or the ‘solar system’, model. Stages to articulate the electron dot formula are stated beneath. Draw the lewis dot structure of a given molecule or ion. Web these diagrams are used as a shorthand notation to show the number of valence electrons in an atom. Draw resonance structures of some molecules. Assess the stability of a structure by considering formal charges of atoms. A beryllium atom, with two valence electrons, would have the electron dot diagram below. Here we have changed the electrons on the hydrogen atoms to be dots. Stages to articulate the electron dot formula are stated beneath. In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. More complicated versions can be used to show the bond between different atoms in a molecule. The first thing we would need to do is to find the total number of valence electrons. Web lewis dot structures, or lewis structures for short, are visuals that represent the outermost shell of electrons, also known as valence electrons, and possible covalent bonds within an atom or molecule. Web a lewis electron dot diagram (or electron dot diagram or a lewis diagram or a lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. This dot and cross diagram shows the outer shells touching. Web an electron configuration diagram is a model that depicts the position of electrons as they orbit the nucleus of an atom. Lewis electron dot diagrams use dots to represent valence electrons around an atomic symbol. Move them around until all the atoms have 8 in their outer orbits. Web electron dot diagrams are diagrams in which the valence electrons of an atom are shown as dots distributed around the element’s symbol. The number of dots equals the number of valence electrons in the atom. Web a lewis structure is a diagram that shows the chemical bonds between atoms in a molecule and the valence electrons or lone pairs of electrons. Use a different color or shape to represent the electrons of each atom.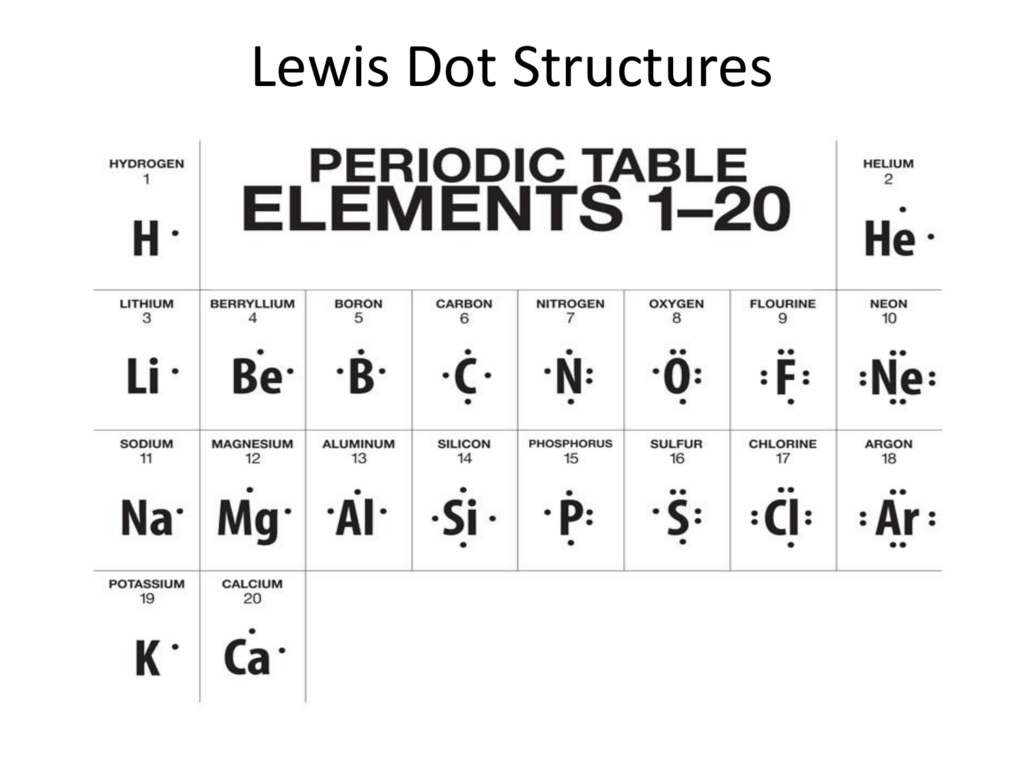
Lewis Dot Structures
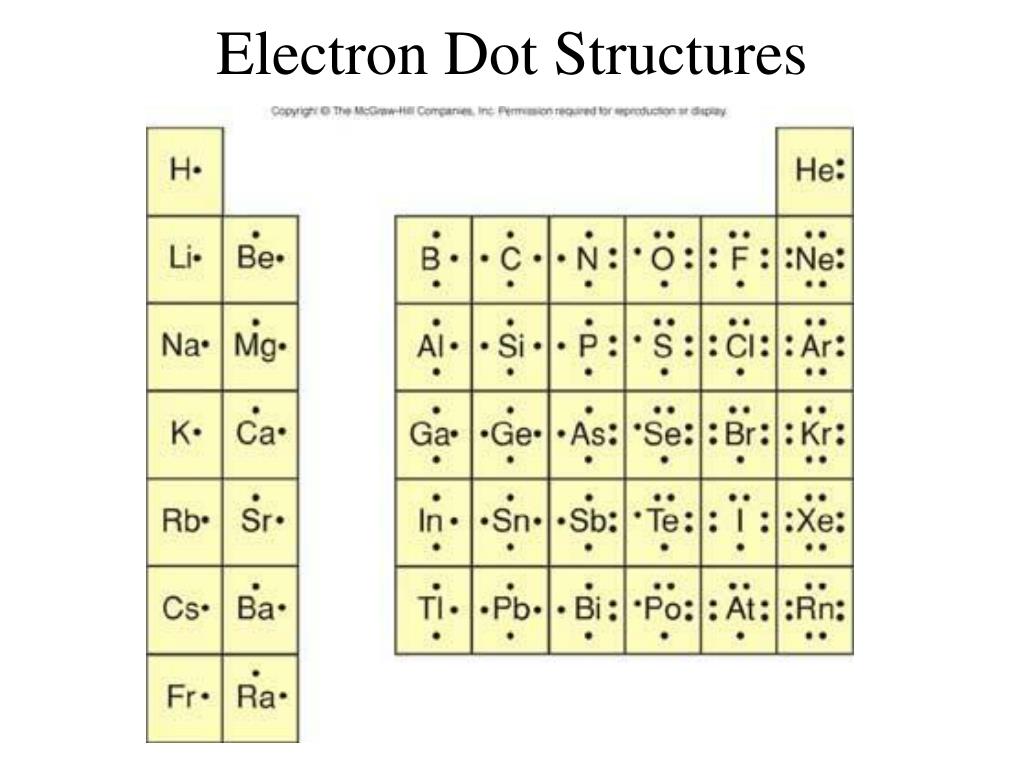
PPT Electron Dot Structures PowerPoint Presentation, free download
/Lewis-dot-structure-58e5390f3df78c5162b4c3db.jpg)
How to Draw a Lewis Structure

Lewis Electron Dot Structure Calculator
How To Draw Lewis Dot Diagrams Simplereality27

Comment dessiner une représentation de Lewis Wiki Chimie

How to Draw a Lewis Structure

Amazing How To Draw An Electron Dot Structure Learn more here

3 Ways to Draw Lewis Dot Structures wikiHow

Lewis Dot Structures of Atoms and Ions YouTube
Web Electron Dot Diagrams Are Diagrams In Which The Valence Electrons Of An Atom Are Shown As Dots Distributed Around The Element's Symbol.
Web Lewis Structures (Also Known As Lewis Dot Structures Or Electron Dot Structures) Are Diagrams That Represent The Valence Electrons Of Atoms Within A Molecule.
To Facilitate Our Understanding Of How Valence Electrons Interact, A Simple Way Of Representing Those Valence Electrons Would Be Useful.
To Facilitate Our Understanding Of How Valence Electrons Interact, A Simple Way Of Representing Those Valence Electrons Would Be Useful.
Related Post: