How Do You Draw An Isotope
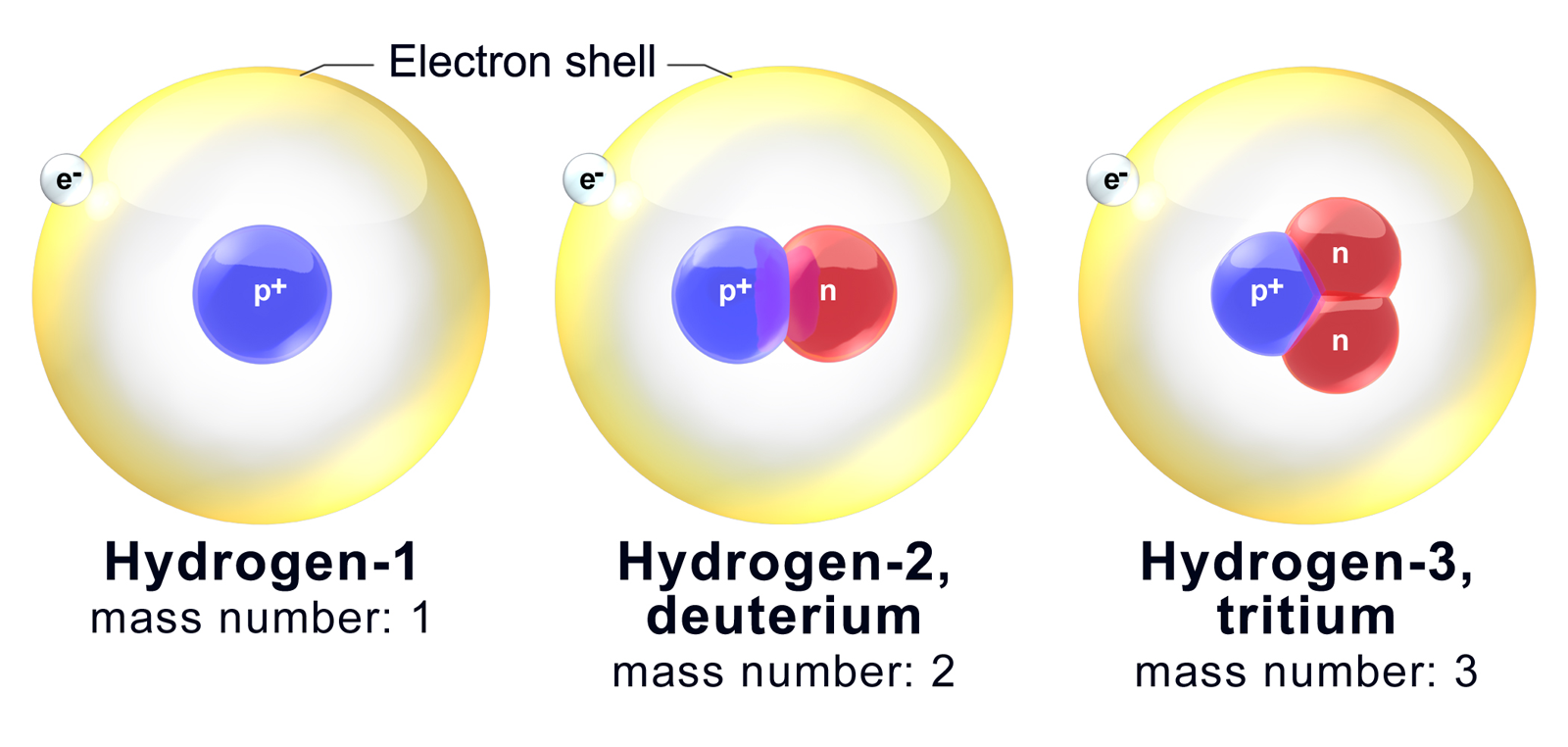
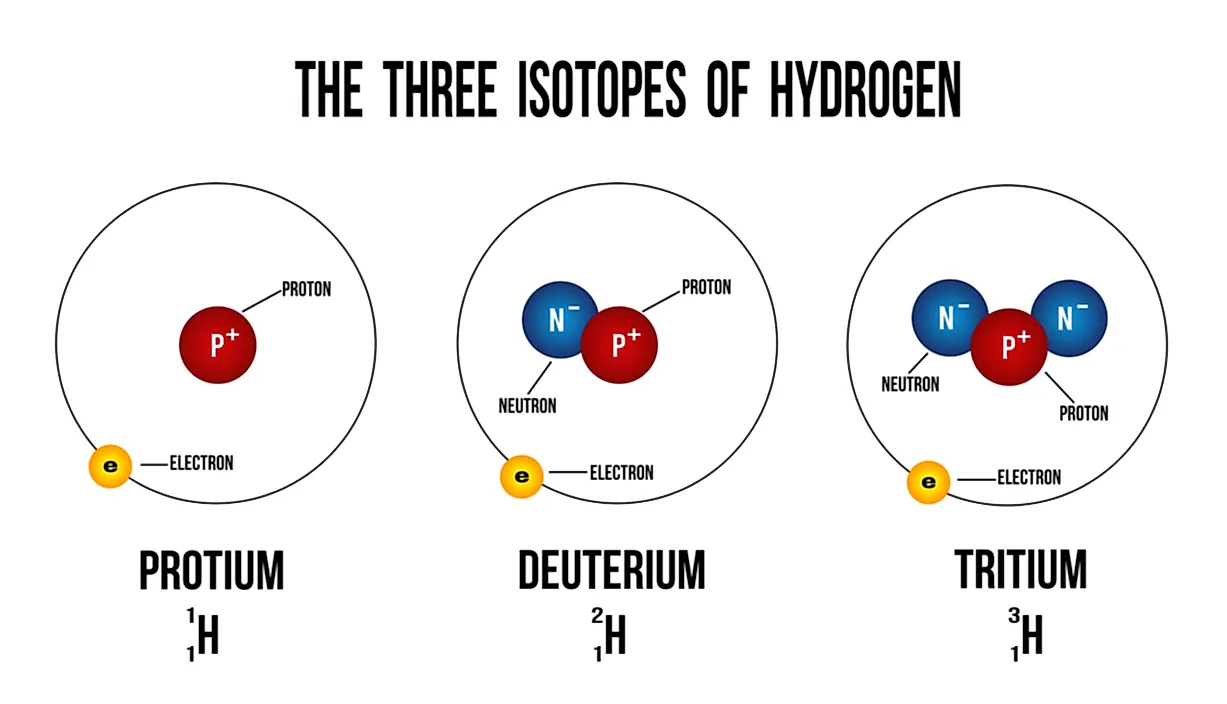
How Do You Draw An Isotope - As mentioned in the previous section, atoms that have the same atomic number (number of protons), but different mass numbers (number of protons and neutrons) are called isotopes (nuclides). Because they contain different numbers of neutrons, isotopes have different atomic masses. Isotopes are atoms of the same element with different numbers of neutrons. Since isotopes of an element have different numbers of neutrons, they also have different masses. Atoms and the periodic table. There are naturally occurring isotopes and isotopes that are artificially produced. For example, a strontium nucleus always has 38 protons, and a rubidium nucleus always has 37. Isotopes are substances that are composed of the. ), but they can contain different numbers of neutrons. It is important to note the difference between an isotope and an elemental symbolism. Web the best way to draw electrons is to draw them as circles with minus signs inside. Isotopes include two or more versions of an atom of a given element that have the same number of protons but a different number of neutrons. Because they contain different numbers of neutrons, isotopes have different atomic masses. Web to write the symbol. Calculate subatomic particles (protons, neutrons, and electrons) for any element by. An isotope symbol communicates information about the element (specifically, the symbol), the atomic number of that element, and the mass number of the specific isotope of the atom. Isotopes are substances that are composed of the. The number of protons (i.e., atomic number, z) determines the element; Web about. Isotopes are atoms of the same element with different numbers of neutrons. 722k views 6 years ago new ap & general chemistry video playlist. The atomic number doesn't change when you're talking about an isotope. Web so there are six neutrons. Isotopes include two or more versions of an atom of a given element that have the same number of. This is carbon and this time we have a superscript of 13. Some elements have only one naturally occurring isotope, but others have two, three or more. The average atomic mass of an element is calculated by taking the weighted average mass of the element's naturally occurring isotopes. Use the number of protons, neutrons, and electrons to draw a model. Isotopes are substances that are composed of the. Isotopes are atoms of the same element with different numbers of neutrons. Web so there are six neutrons. The symbols for the two naturally occurring isotopes of chlorine then would be. The sum of the number of neutrons and protons in. The symbols for the two naturally occurring isotopes of chlorine then would be. Isotopes are atoms of the same element with different numbers of neutrons. Web so there are six neutrons. This is carbon and this time we have a superscript of 13. ), but they can contain different numbers of neutrons. If you change the atomic number, you change the element. The sum of the number of neutrons and protons in. Additionally, n = a −z. All hydrogen atoms contain one proton (and one. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. The sum of the number of neutrons and protons in. Then play a game to test your ideas! Web isotope notation, also known as nuclear notation, is important because it allows us to use a visual symbol to easily determine an isotope's mass number, atomic number, and to determine the number of neutrons and protons in the nucleus without having. Isotopes include two or more versions of an atom of a given element that have the same number of protons but a different number of neutrons. Some elements have only one naturally occurring isotope, but others have two, three or more. 92k views 5 years ago. Additionally, n = a −z. This indicates not only that they are electrons, but. It is important to note the difference between an isotope and an elemental symbolism. Calculate subatomic particles (protons, neutrons, and electrons) for any element by. Isotopes are substances that are composed of the. The number of protons (i.e., atomic number, z) determines the element; Then, at several later times, the procedure is repeated and the new fraction of various isotopes. If you change the atomic number, you change the element. Since isotopes of an element have different numbers of neutrons, they also have different masses. 722k views 6 years ago new ap & general chemistry video playlist. How to draw an atom! Web we recommend using the latest version of chrome, firefox, safari, or edge. An isotope symbol communicates information about the element (specifically, the symbol), the atomic number of that element, and the mass number of the specific isotope of the atom. Atoms and the periodic table. It is important to note the difference between an isotope and an elemental symbolism. 92k views 5 years ago. ), but they can contain different numbers of neutrons. Because they contain different numbers of neutrons, isotopes have different atomic masses. Then, at several later times, the procedure is repeated and the new fraction of various isotopes is recorded. The symbols for the two naturally occurring isotopes of chlorine then would be. There are naturally occurring isotopes and isotopes that are artificially produced. The number of protons (i.e., atomic number, z) determines the element; For example, a strontium nucleus always has 38 protons, and a rubidium nucleus always has 37.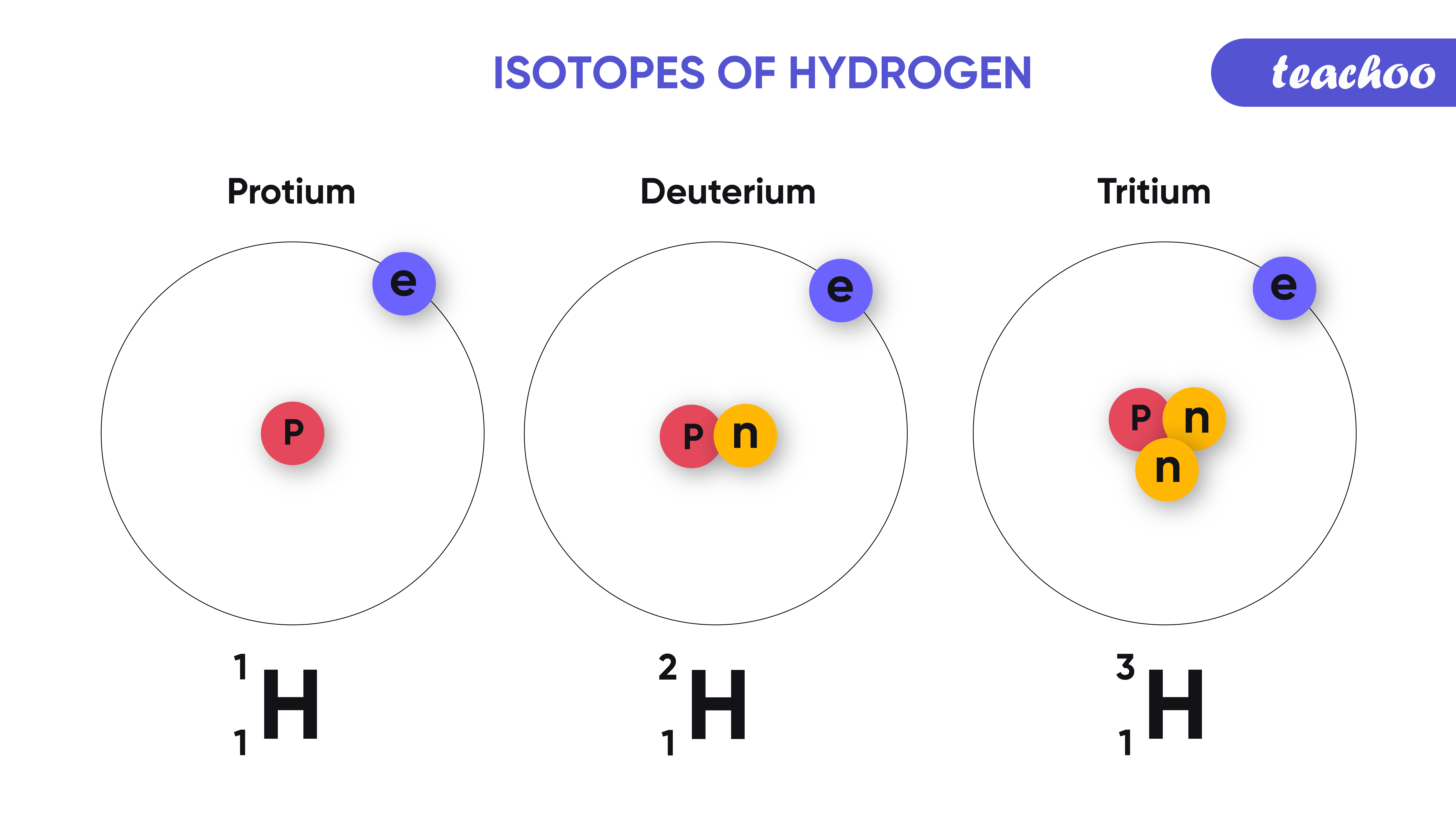
Isotopes and Isobars Definition, Uses and Difference Teachoo
/Isotope-58dd6b415f9b5846830254ae.jpg)
Isotopes Definition and Examples in Chemistry

Isotopes What are Isotopes? Relative Atomic Mass

Explain Why There Are Different Isotopes for a Given Element Baylee

How Do the Isotopes Hydrogen 2 and Hydrogen 3 Differ

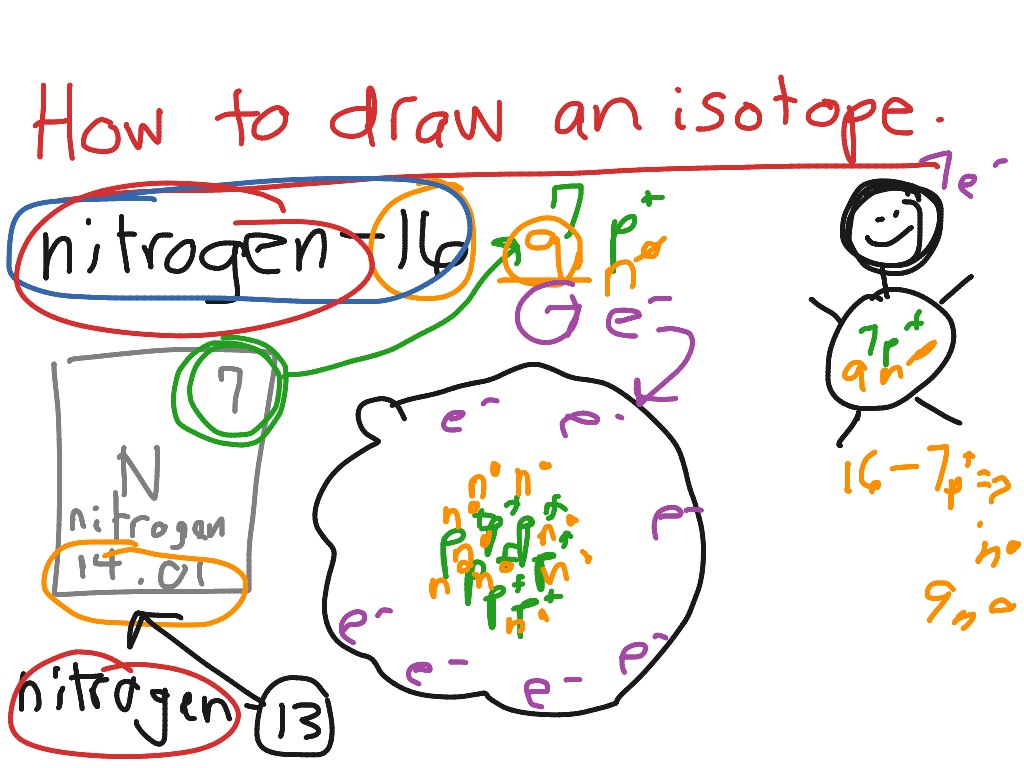
How to draw an isotope YouTube
DOE Explains...Isotopes Department of Energy
How to Draw an Isotope Science ShowMe
What is an Isotope? The Knowledge Library

Isotopes — Definition & Overview Expii
[ Show Transcript ] Subscribe To Jefferson Lab's Youtube Channel And Be Notified When We Post New Videos!
Isotopes Are Atoms Of The Same Element With Different Numbers Of Neutrons.
Use The Number Of Protons, Neutrons, And Electrons To Draw A Model Of The Atom, Identify The Element, And Determine The Mass And Charge.
Web How To Write An Isotope.
Related Post: