Note To File Template Clinical Research
Note To File Template Clinical Research - Need assistance or have clinical study management questions? Please note that this page has been updated for 2015 following a quality check and review of the templates, and many new ones have been added. It is used to clarify an error, omission or discrepancy or to document a problem or corrective action. When left unchecked, the ntfs multiply to fill the trial master file, or tmf. Web note to file template. March 31, 2010 this subject was consented by dr. Web note to file. Standard operating procedure (sop) template doc. Joe brown, research coordinator [insert staff name, include role on study} to: The study management templates are adaptable and downloadable templates that can be used to organize and maintain research study documentation as you would include in a regulatory binder. Record retention flow chart pdf. Web for clinical researchers, the note to file, or ntf, is the mosquito that you can hear under the covers or the flat tire on the way to the interview. Joe brown, research coordinator [insert staff name, include role on study} to: It is used to clarify an error, omission or discrepancy or to document. List of standard operating procedures (sops) required at clinical research sites pdf. This article explores this evolution from the perspective of an independent observer. Record retention flow chart pdf. The primary purpose of every tmf is to tell the story of a clinical trial. Score manual appendix note to filde template last modified by: This guidance provides a description of a note to file, when they should be used as a documentation practice during the conduct of human subjects research. Document and address any issue that is protocol and/or site specific that cannot be resolved without a change from previously approved procedures; Retained fluid and tissue log. Web note to file template to be. Retained fluid and tissue log. Back to table of contents. Web clinical trialists, template, templates. Regulatory file/ investigator site file. The primary purpose of every tmf is to tell the story of a clinical trial. Animal care and use award management conflicts of interest contracts and subawards controlled substances environmental health and safety export control hipaa human stem cell research human subjects research. Standard operating procedure (sop) template doc. Need assistance or have clinical study management questions? Document and address any issue that is protocol and/or site specific that cannot be resolved without a change. The study management templates are adaptable and downloadable templates that can be used to organize and maintain research study documentation as you would include in a regulatory binder. Web note to file. Web note to file template to be used to create a note to file which are written to identify a discrepancy or problem in the conduct of the. Record retention flow chart pdf. [insert date of the ; Ote to file (ntf)] clinical research site. Web guidance for creating a note to file. Need assistance or have clinical study management questions? March 31, 2010 this subject was consented by dr. If the issue relates to site performance, the appropriate credentialed individual from the site should write and sign the note to file. Notes to the study file (ntf) are written to. Web note to file template to be used to create a note to file which are written to identify a. The primary purpose of every tmf is to tell the story of a clinical trial. Web welcome to global health trials' tools and templates library. Web valid notes to file (ntfs) should, at minimum, meet the following basic criteria: The study management templates are adaptable and downloadable templates that can be used to organize and maintain research study documentation as. Notes to the study file are written to identify a discrepancy or problem in the conduct of the clinical research study, note the root cause of the identified problem, identify the corrective action taken to prevent recurrence of the problem, and document that the corrective action has resolved the problem. Web also, the “note to file” dated 7/10/09 for subject. Notes to the study file (ntf) are written to. The story of a clinical trial must relay a powerful narrative about the production of quality scientific data and the protection of patient’s rights and safety. Notes to the study file are written to identify a discrepancy or problem in the conduct of the clinical research study, note the root cause of the identified problem, identify the corrective action taken to prevent recurrence of the problem, and document that the corrective action has resolved the problem. Protocol number, version and date: The study management templates are adaptable and downloadable templates that can be used to organize and maintain research study documentation as you would include in a regulatory binder. List of standard operating procedures (sops) required at clinical research sites pdf. Web note to file template. Please note that this page has been updated for 2015 following a quality check and review of the templates, and many new ones have been added. Score manual appendix note to filde template last modified by: Web guidance for creating a note to file. Record retention flow chart pdf. Retained fluid and tissue log. Web welcome to global health trials' tools and templates library. Standard operating procedure (sop) template doc. Web a note to the study file should be printed on institution letterhead and should be initiated and authored by the individual or organization responsible for its content, as follows: Web note to file template doc.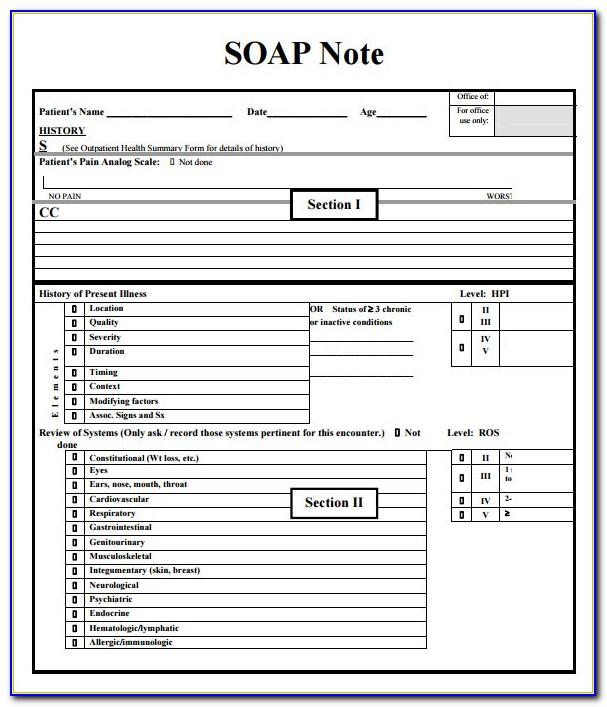
Clinical Progress Note Format
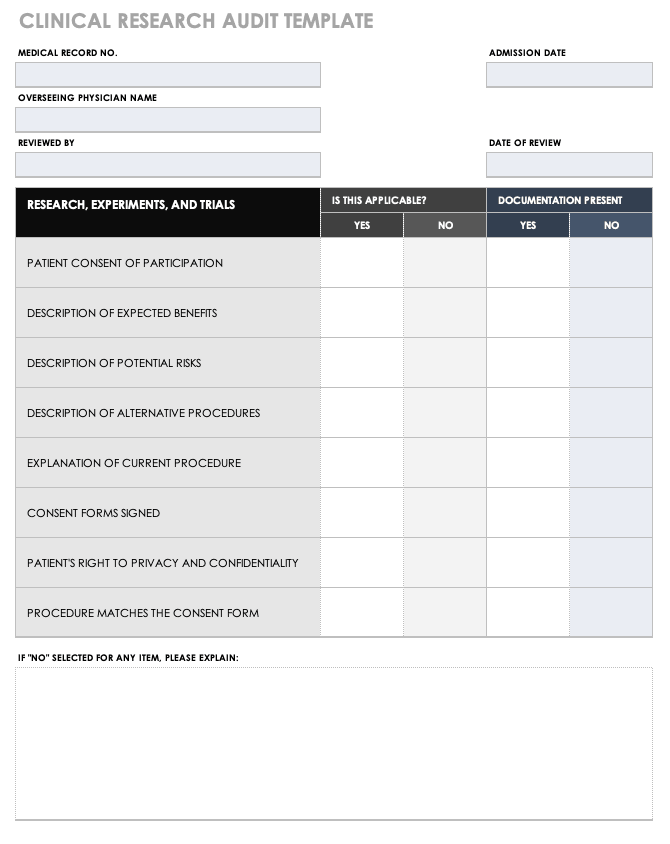
Free Medical Form Templates Smartsheet

Medical Note Template Free Word Template, Format & Example

Note To File Template Download by Pharma Student Issuu

Clinical Progress Note How to create a Clinical Progress Note

Irb Protocol Template

Psychiatric H&P Template
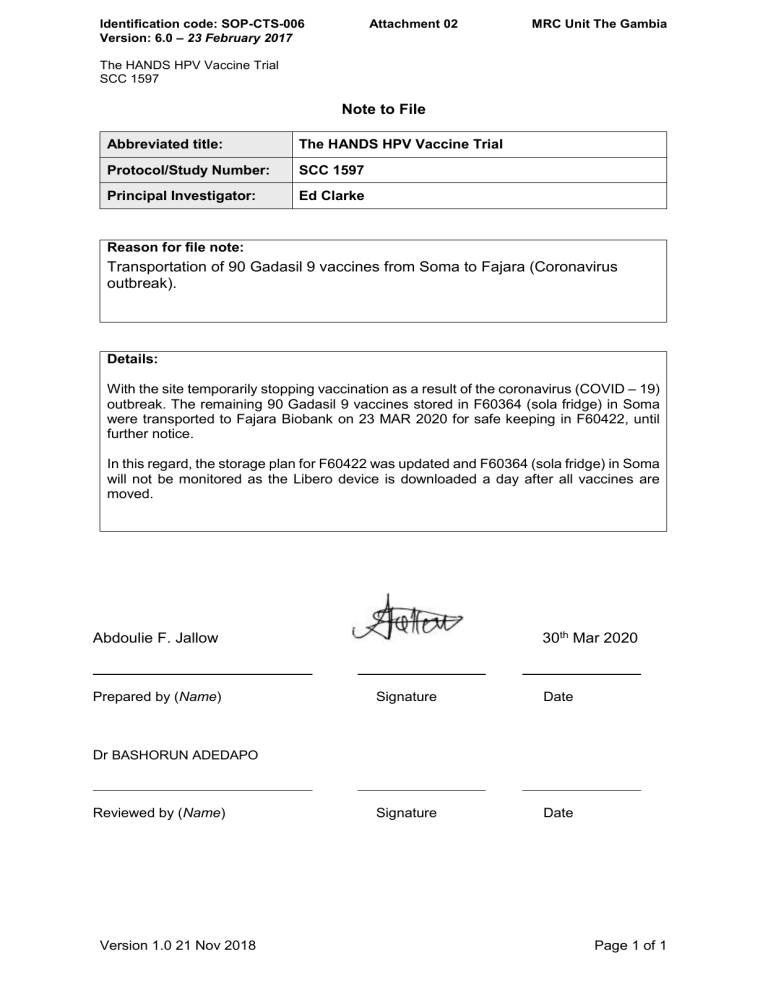
File Note Template

Clinical Trial Compliance Using Notes to File to Document Variation

Clinic Note Templates Clinic Transcription Internal Medicine
Document And Address Any Issue That Is Protocol And/Or Site Specific That Cannot Be Resolved Without A Change From Previously Approved Procedures;
Web Also, The “Note To File” Dated 7/10/09 For Subject (B) (6) Indicates That The Subject Completed All Screening Assessments On 2/14/2008 And That You Did Not Obtain Informed Consent Until 2/15/2008.”.
Clinical Quality Management Plan Template Doc.
Web Clinical Trialists, Template, Templates.
Related Post: