Sigma Bond Drawing
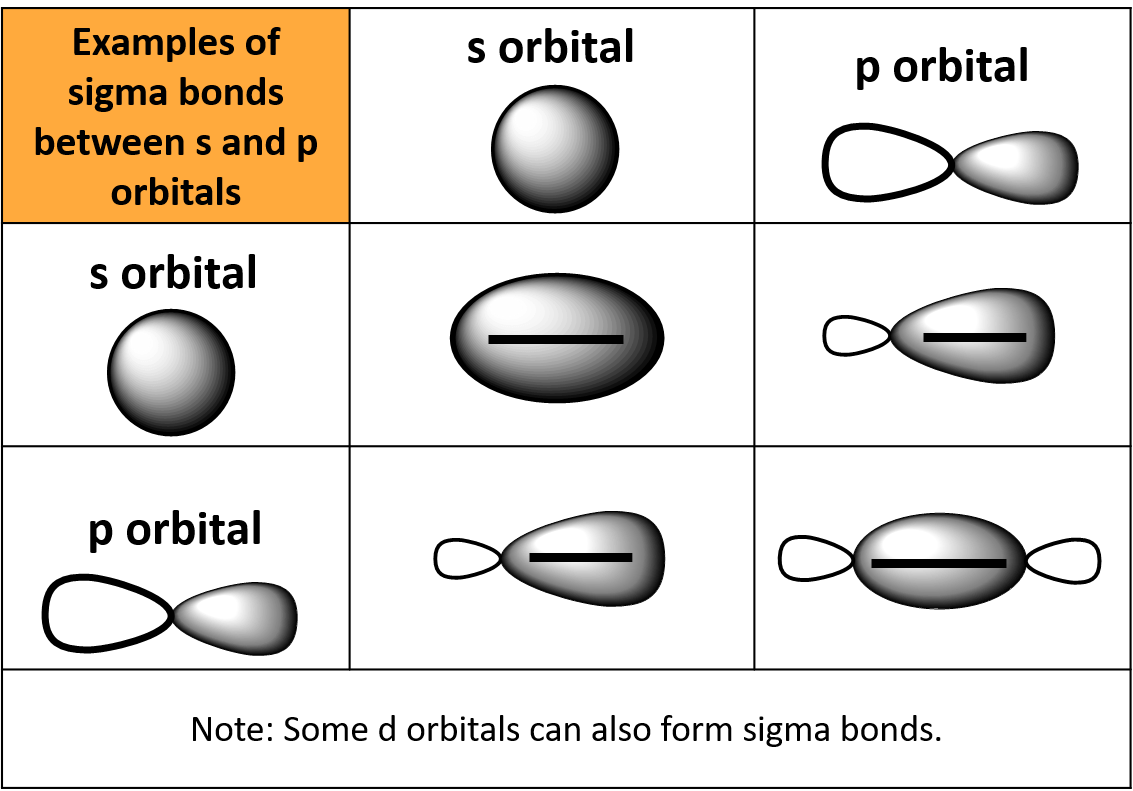
Sigma Bond Drawing - An sp orbital is composed of one s orbital and one p orbital, and thus it has 50% s character and 50% p character. 9.4 sigma and pi bonds | general chemistry. Web the figure below illustrates the sigma and pi bonds in an ethylene molecule (\(c_2h_4\)). The pi bond is the second bond of the double bonds between the carbon atoms, and is shown as an elongated green lobe that extends both above and below the plane of the molecule. Thus we need to leave one electron (in case of carbon double bond) to let the carbon have the second bond as a. Describe the formation of covalent bonds in terms of molecular orbitals. Π bonds are only found within double and triple bonds. Web this is called a sigma bond, where the overlap is along the same axis as if you connected the two molecules. Label the positions of the oxygen nuclei with the symbol o. To start, we must explain both bonds: The hybridization model can explain covalent bond formation in a molecule. Between pi bond and sigma bond, which bond can be broken easily and why? Web how can i draw sigma bonds? 1.1m views 6 years ago new ap & general chemistry video playlist. Account for differences in bond length and strength in terms of the efficiency with which atomic. The bond between two hydrogen atoms is an example of sigma bonding. The two clouds of electrons in a π bond represent one bond containing two electrons. Likewise, a triple bond consists of one sigma bond and two pi bonds. Web when orbitals approach each other in a head to head fashion, the resulting covalent bonds are called sigma bonds.. Web sigma and pi bonds are an aspect of valence bond theory and molecular orbital theory that explains the existence of double and triple bonds. Web all three are single (sigma) bonds; The electron density is concentrated on opposite sides of the bond axis. Web in chemistry, sigma bonds ( σ bonds) are the strongest type of covalent chemical bond.. The two clouds of electrons in a π bond represent one bond containing two electrons. 1.1m views 6 years ago new ap & general chemistry video playlist. Sigma bonds form when the available orbital with the highest energy of each atom overlaps one another. Thus we need to leave one electron (in case of carbon double bond) to let the. Web sigma and pi bonds are an aspect of valence bond theory and molecular orbital theory that explains the existence of double and triple bonds. To start, we must explain both bonds: The hybridization model can explain covalent bond formation in a molecule. Account for differences in bond length and strength in terms of the efficiency with which atomic orbitals. With this information, you can easily count sigma and pi bonds. To count sigma and pi bonds, draw the lewis dot structure and count the single, double and triple bonds present. The pi bond is the second bond of the double bonds between the carbon atoms, and is shown as an elongated green lobe that extends both above and below. As illustrations, consider the bonds that have already been studied. Web #charusscienceworld drawing of sigma and pi bonds formation in n2 molecule Between pi bond and sigma bond, which bond can be broken easily and why? After completing this section, you should be able to. This chemistry video tutorial provides a basic introduction into sigma and pi bonds. An sp orbital is composed of one s orbital and one p orbital, and thus it has 50% s character and 50% p character. 1.1m views 6 years ago new ap & general chemistry video playlist. Over here, you connect the two molecules, the overlap is on that same axis. The explanation is relatively straightforward. The bond between two hydrogen. As illustrations, consider the bonds that have already been studied. Sigma bonds form when the available orbital with the highest energy of each atom overlaps one another. Sigma bonding is most simply defined for diatomic molecules using the language and tools of. Thus we need to leave one electron (in case of carbon double bond) to let the carbon have. To count sigma and pi bonds, draw the lewis dot structure and count the single, double and triple bonds present. The pi bond is the second bond of the double bonds between the carbon atoms, and is shown as an elongated green lobe that extends both above and below the plane of the molecule. Web how can i draw sigma. The bond between two hydrogen atoms is an example of sigma bonding. The electron density is concentrated on opposite sides of the bond axis. The bond in propyne is shortest and strongest, while the bond in propane is longest and weakest. Account for differences in bond length and strength in terms of the efficiency with which atomic orbitals overlap. This chemistry video tutorial provides a basic introduction into sigma and pi bonds. Web #charusscienceworld drawing of sigma and pi bonds formation in n2 molecule This is the strongest form of covalent bonds, and this'll be a good basis for discussion maybe in the next video when we talk a little bit about pi bonds. Web the figure below illustrates the sigma and pi bonds in an ethylene molecule (\(c_2h_4\)). With this information, you can easily count sigma and pi bonds. In contrast, pi ( π) bonds, which are formed when π molecular orbitals are made, are found in double (one σ + one π bond) and triple (one σ + two π bonds) bonds. The diagram below (figure 3) is a representation of the energy levels of the bonding and antibonding orbitals formed in the hydrogen molecule. A sigma bond is the first bond between two atoms, and is the strongest sort of covalent bond. The two bonds differ in the way in which overlapping occurs. How to count sigma and pi bonds? And a hybridized orbital cannot be involved in a pi bond. The pi bond is the second bond of the double bonds between the carbon atoms, and is shown as an elongated green lobe that extends both above and below the plane of the molecule.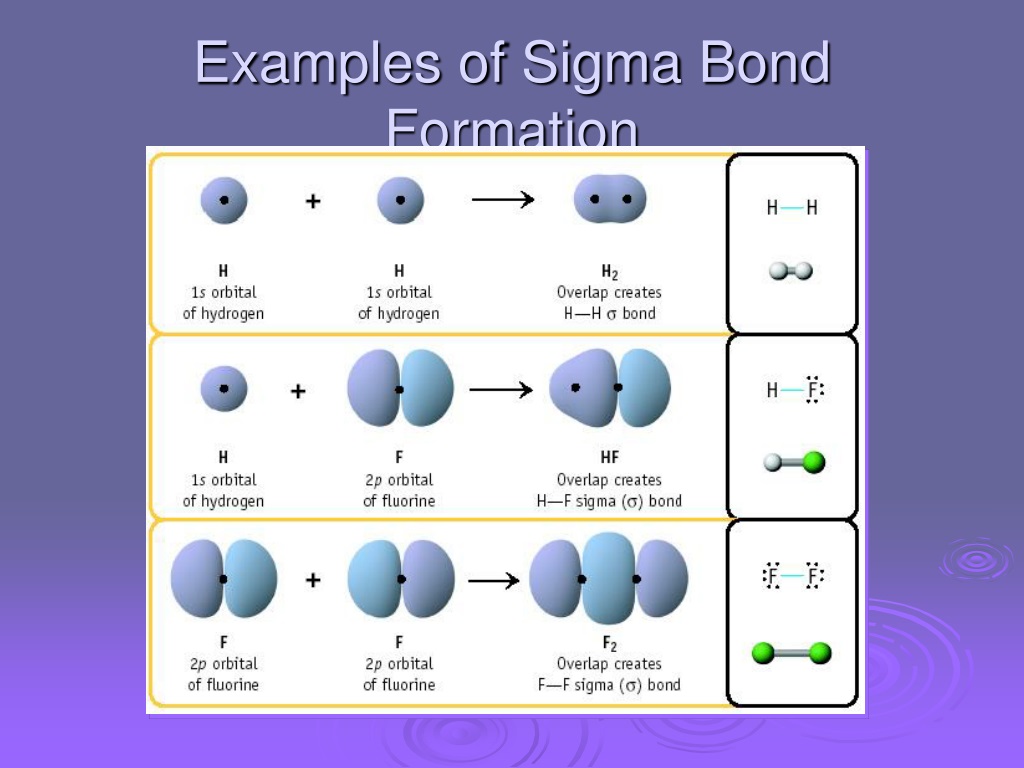
Sigma bond gilitmetro

Sigma and Pi Bonds Brilliant Math & Science Wiki
Sigma (δ) and Pi ((π) Bond (ALevel) ChemistryStudent
Sigma and Pi Bonds — Definition & Overview Expii
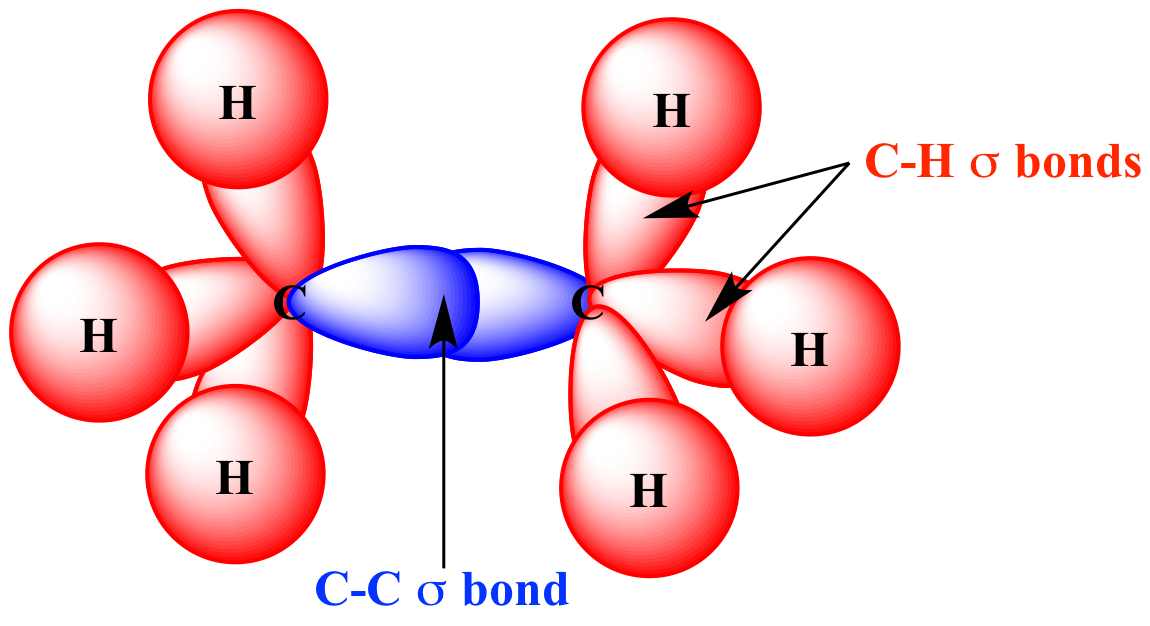
Illustrated Glossary of Organic Chemistry Sigma bond (σ bond)
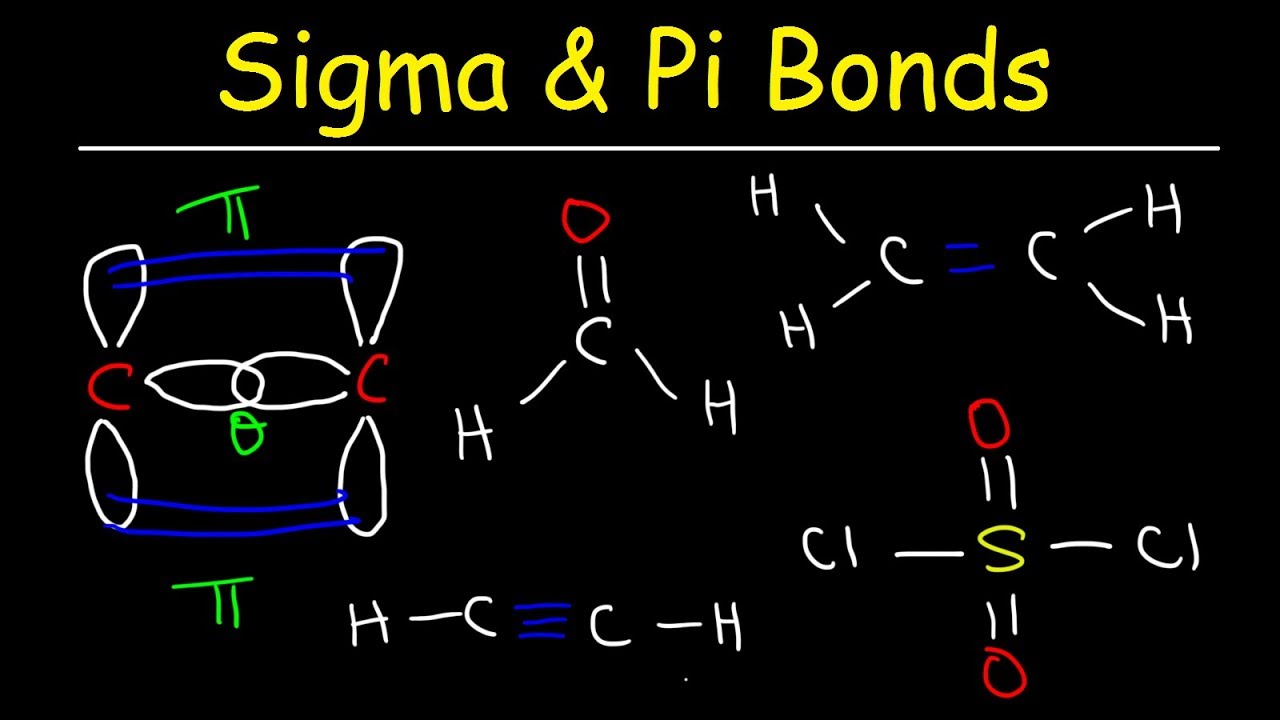
Sigma and Pi Bonds Explained, Basic Introduction, Chemistry Inflation
Why are sigma bond more stronger than pi bond ? PG.CHEMEASY
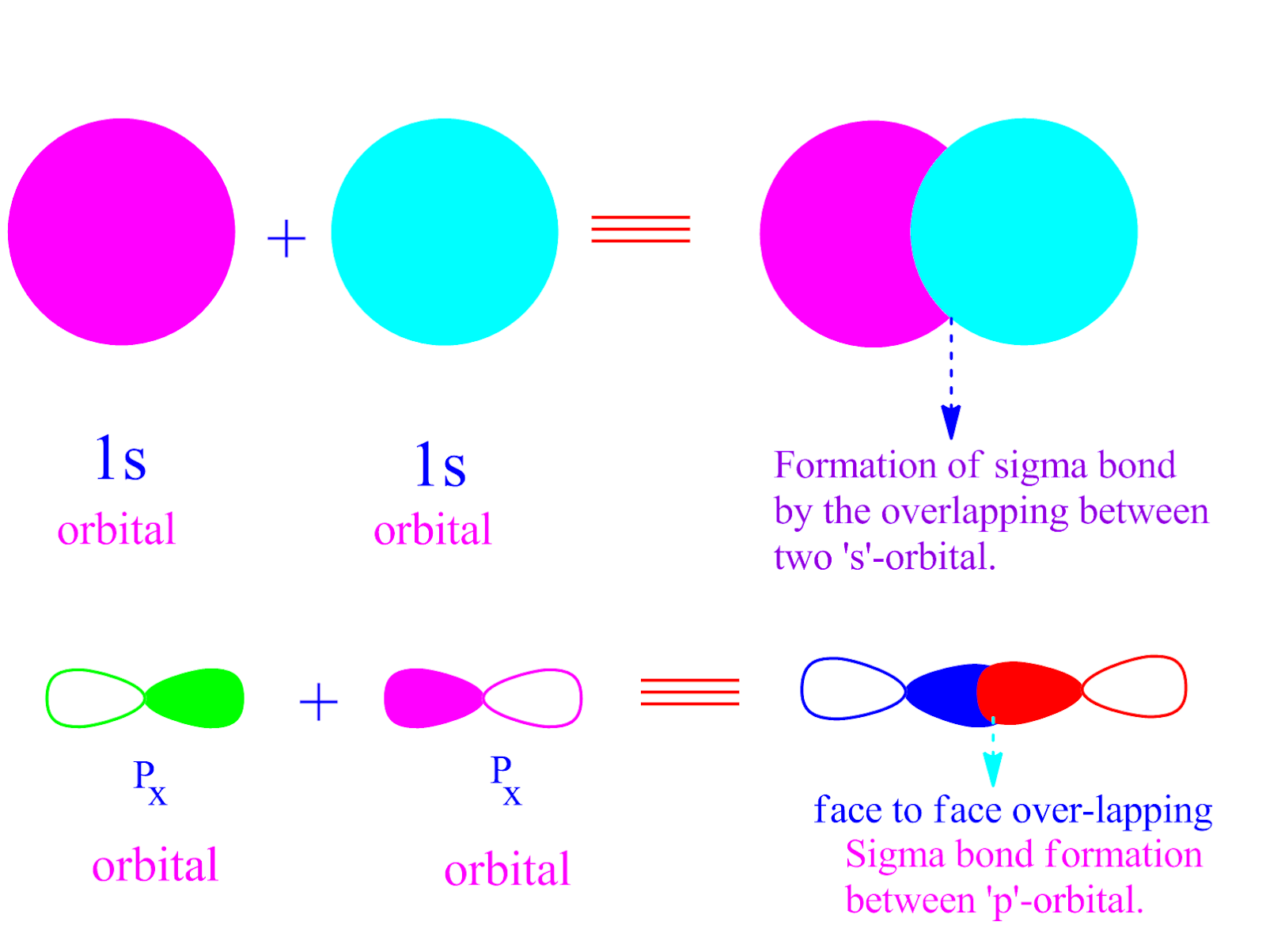
[Solved] sketch sigma and pi bond from p orbital Course Hero

8 Drawing Molecular Orbital Diagrams — Flux Science

Draw sigma and pi bonds YouTube
Web Drawing Lewis Structures With Multiple Bonds.
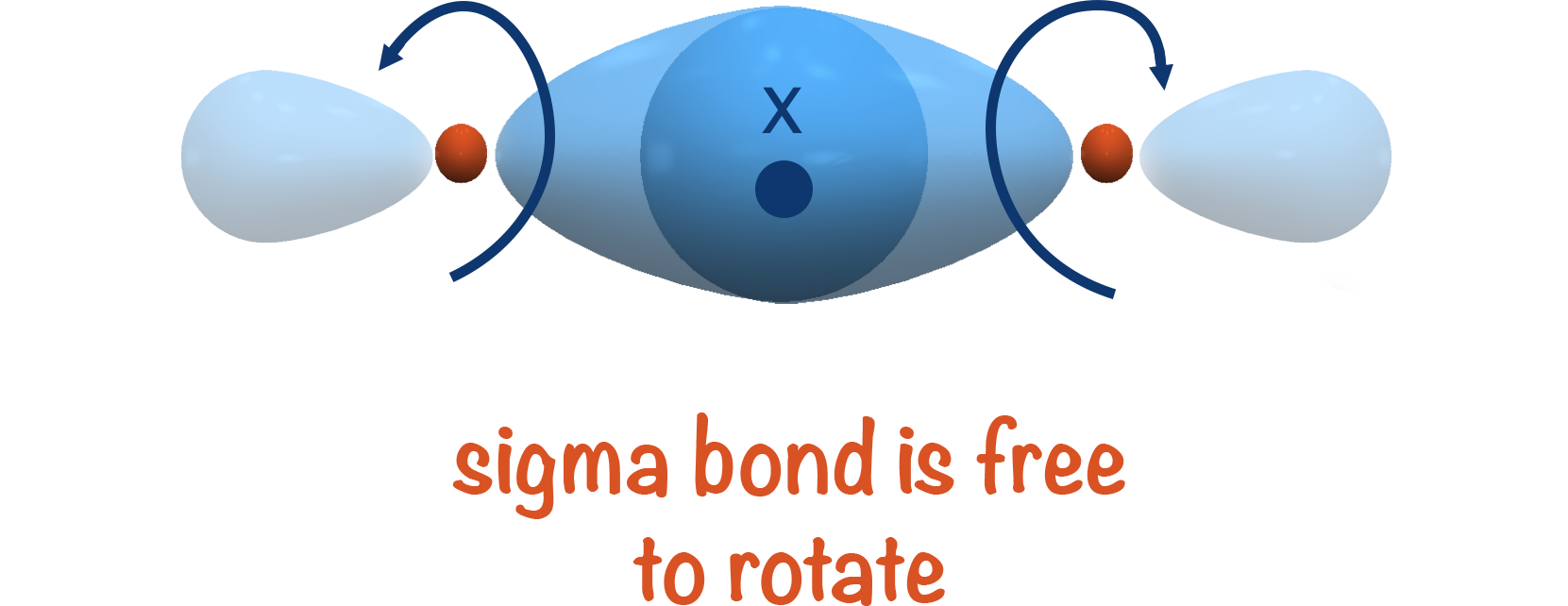
Web This Is Called A Sigma Bond, Where The Overlap Is Along The Same Axis As If You Connected The Two Molecules.
To Start, We Must Explain Both Bonds:
Π Bonds Are Only Found Within Double And Triple Bonds.
Related Post: