Validation Master Plan Template
Validation Master Plan Template - Purpose of the validation this will usually be to ensure that the facility or equipment being validated conforms to the Web three (3) options to create a validation master plan. 2.1 purpose of the document. Web critical components of a vmp. Web what is a validation master plan template? Web a validation master plan (vmp) is a documented plan that outlines the overall strategy and approach for validation activities within a pharmaceutical or medical device manufacturing facility. Web at the core of the validation process is a fundamental document known as a validation master plan (vmp). Web a validation master plan, often referred to as vmp, is a comprehensive document that defines and provides evidence of an organization’s commitment towards quality assurance. Web a quick validation master plan checklist. Validation master plan from the institute of validation technology (note: The purpose of this document is to record the schedule for conducting the validations and record the status and accomplishment of. To see the complete list of the most popular validation templates, click here. How to creation one validation master plot before the next fda audit? Home › complianceonline standards › fda validation › validation master plan template. Whether you're. Web validation master plan template document control details this will include details such as vmp reference number, version number, date and authorisation signatures. Web a validation master plan (vmp) outlines the principles involved in the qualification of a facility, defining the areas and systems to be validated, and provides a written program for achieving and maintaining a qualified facility. Web. 5.2.7 for large projects involving many materials, a materials validation plan may be used. Purpose of the validation this will usually be to ensure that the facility or equipment being validated conforms to the Facilitate fda inspections with a validation master plan. Web master validation plans not a specific 820 requirement, but is recommended per ghtf guidance. Web a validation. The four recommendations comprising this document define general principles pertaining to each of the topics. Senior gxp regulatory compliance expert. You can create a great protocol, using a template. Identify what needs to be validated iii. The purpose of this document is to record the schedule for conducting the validations and record the status and accomplishment of. Teaching more & transfer a free sample here Web the validation master plan is a summary of validation strategy. Web a validation master plan, also referred to as “vmp”, outlines the principles involved in the qualification of a facility, defining the areas and systems to be validated, and provides a written program for achieving and maintaining a qualified facility. Identify. The vmp (validation master plan) or lower tier documentation alone may cover the The purpose of this document is to record the schedule for conducting the validations and record the status and accomplishment of. Validation master plan from the institute of validation technology (note: Whether you're setting out to develop a vmp or seek to identify weaknesses in an existing. Tips for writing a validation master plan. Web validation master plan template. The vmp (validation master plan) or lower tier documentation alone may cover the Identify what needs to be validated iii. Web three (3) options to create a validation master plan. The plan should also reference all applicable protocols and reports related to each included item. The following content can be applied to a mvp: Web validation master plan template document control details this will include details such as vmp reference number, version number, date and authorisation signatures. Web what is a validation master plan template? Web validation master plan template. In this comprehensive guide, we’ll address key questions such as “what does a validation master plan include?” and “how can i create a vmp?” and “who is responsible for preparing a vmp?” Purpose of the validation this will usually be to ensure that the facility or equipment being validated conforms to the This master plan describes the risk analysis approach. Home › complianceonline standards › fda validation › validation master plan template. Web master validation plan is a strategic document which identifies the elements to be validated, the approach to be taken for validation of each element, the organizational responsibilities and the documentation to be produced in order to ensure full consideration is given to product quality aspects. Senior gxp. Web the purpose of the validation master plan template (vmp) is to describe the organization’s overall strategy, approach, and responsibilities for validation of computer systems and software. Identify what needs to be validated iii. Web a validation master plan, also referred to as “vmp”, outlines the principles involved in the qualification of a facility, defining the areas and systems to be validated, and provides a written program for achieving and maintaining a qualified facility. Define the product and process flow ii. Home › complianceonline standards › fda validation › validation master plan template. The four recommendations comprising this document define general principles pertaining to each of the topics. 5.2.7 for large projects involving many materials, a materials validation plan may be used. Web three (3) options to create a validation master plan. Tips for writing a validation master plan. Web master validation plans not a specific 820 requirement, but is recommended per ghtf guidance. It acts as a cornerstone for the validation process, serving as an operational guide detailing the implementation and validation of systems and equipment in your organization. Senior gxp regulatory compliance expert. The vmp (validation master plan) or lower tier documentation alone may cover the In this comprehensive guide, we’ll address key questions such as “what does a validation master plan include?” and “how can i create a vmp?” and “who is responsible for preparing a vmp?” A validation master plan summarizes all required people, systems equipment, timing, and processes. As you may know, vaisala offers several web tutorials on validation throughout the year.
How to create a Validation Master Plan in 5 steps. Templates & more

Master Validation Plan Template
Validation Master Plan
Master Validation Plan Template
Validation Master Plan (VMP) Downloadable Interactive Template.
What Is Validation Master Plan Template Examples vrogue.co
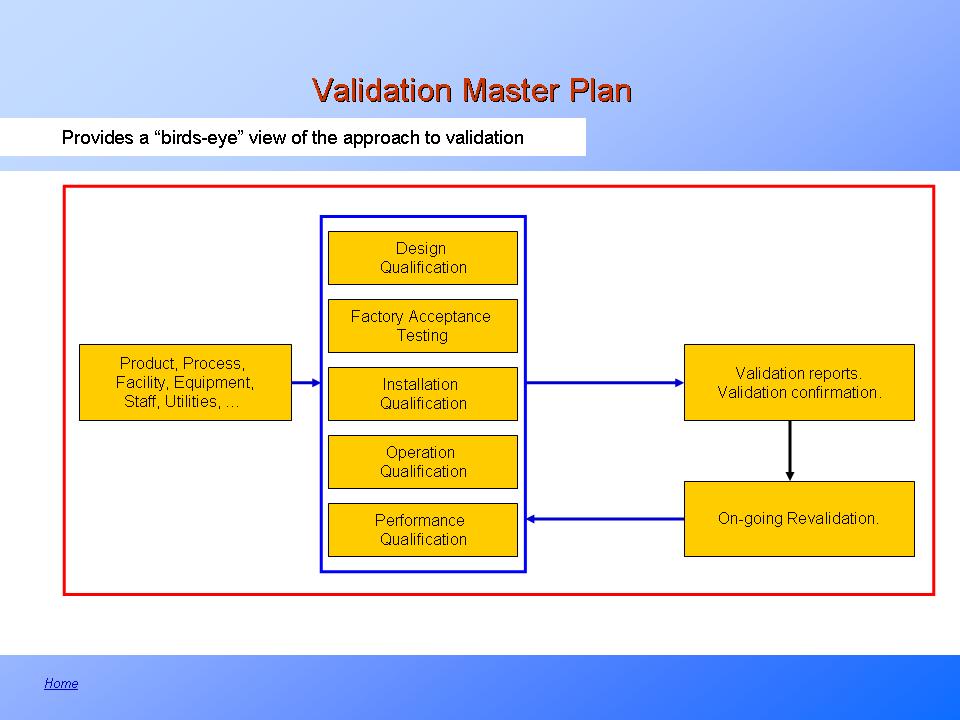
Overview of the Validation Master Plan PresentationEZE

Validation Master Plan Template Validation Center

Sample Validation Plan Template 9+ Free Documents in PDF, Word
Validation Master Plan Template Verification And Validation
Web A Validation Master Plan (Vmp) Is A Documented Plan That Outlines The Overall Strategy And Approach For Validation Activities Within A Pharmaceutical Or Medical Device Manufacturing Facility.
Web What Is A Validation Master Plan Template?
Teaching More & Transfer A Free Sample Here
Web A Validation Master Plan, Often Referred To As Vmp, Is A Comprehensive Document That Defines And Provides Evidence Of An Organization’s Commitment Towards Quality Assurance.
Related Post:
