Hydrogen Bond Drawing
Hydrogen Bond Drawing - The phosphate group, the deoxyribose sugar, and the nitrogenous base. Hydrogen bonds are intermolecular forces; A possible hydrogen bond is defined by the following criteria: Ionic bonds form when one atom transfers electrons to another atom. Intermolecular bonds are bonds between molecules. These properties allow cells to regulate their internal temperature, provide lubrication, and facilitate nutrient uptake and waste removal. 10k views 3 years ago #exploding #teacher #chemical. Covalent and ionic bonds are intramolecular forces. Polar molecules, such as water molecules, have a weak, partial negative charge at one region of the molecule (the oxygen atom in water) and a partial positive charge elsewhere (the hydrogen atoms in water). Carbon is still bonded to these hydrogens but we're going to ignore them for our bond line structure. The partial negative charge on the o of one molecule can form a hydrogen bond with the partial positive charge on the hydrogens of other molecules. A possible hydrogen bond is defined by the following criteria: Intermolecular forces (imfs) occur between molecules. Web hydrogen bonds between water molecules give water its high boiling point, high heat capacity, and surface tension.. Hydrogen bonds can exist between atoms in different molecules or in parts of the same molecule. Web amea is now turning its experience to the production of green hydrogen in egypt. It’s project, announced at cop27, will have a 500mw electrolyser and ammonia production plant built in the sczone. Web explain what is meant by hydrogen bonding and the molecular. Web hydrogen bonding, interaction involving a hydrogen atom located between a pair of other atoms having a high affinity for electrons; You need to remember that each line represents a pair of shared electrons. Hydrogen bonding is the strongest form of intermolecular bonding. A with no hydrogen bonded to it, a hydrogen bond exists between. Hydrogen bonds can exist between. A possible hydrogen bond is defined by the following criteria: Sketch out structural examples of hydrogen bonding in three small molecules other than h 2 o. Web there are various ways of drawing this and you will need to be familiar with all of them. Also, helium is shown in group 8a, but it only has two valence electrons. Intermolecular. The atom that loses an electron becomes a positive ion. Hydrogen bonding is the strongest form of intermolecular bonding. 10k views 3 years ago #exploding #teacher #chemical. Web hydrogen bonding, interaction involving a hydrogen atom located between a pair of other atoms having a high affinity for electrons; The phosphate group, the deoxyribose sugar, and the nitrogenous base. Be sure to learn them well and avoid them in your answers! The molecules which have this extra bonding are: Covalent and ionic bonds are intramolecular forces. The origin of hydrogen bonding. Web there are various ways of drawing this and you will need to be familiar with all of them. Hydrogen bonding is also vital for the structure and stability of biomolecules. Web explain what is meant by hydrogen bonding and the molecular structural features that bring it about. Web these relatively powerful intermolecular forces are described as hydrogen bonds. Web head of science. The hbond representation will draw a dotted line between two atoms if there is a possible. Web head of science. Web if you were to draw every carbon hydrogen bond in organic chemistry class it would take you forever. You need to remember that each line represents a pair of shared electrons. This video shows three examples of drawing for the formation of hydrogen bond. Hydrogen bonding is the strongest form of intermolecular bonding. It’s project, announced at cop27, will have a 500mw electrolyser and ammonia production plant built in the sczone. A with no hydrogen bonded to it, a hydrogen bond exists between. For hydrogen bonding to take place the following is needed: The molecules which have this extra bonding are: This video shows three examples of drawing for the formation of hydrogen. The origin of hydrogen bonding. For hydrogen bonding to take place the following is needed: Web water molecules forming hydrogen bonds with one another. The phosphate group, the deoxyribose sugar, and the nitrogenous base. Hydrogen bonds can exist between atoms in different molecules or in parts of the same molecule. How are hydrogen bonds different from covalent and ionic bonds? The partial negative charge on the o of one molecule can form a hydrogen bond with the partial positive charge on the hydrogens of other molecules. You need to remember that each line represents a pair of shared electrons. Describe the roles of hydrogen bonding in proteins and in dna. Web these relatively powerful intermolecular forces are described as hydrogen bonds. Web explain what is meant by hydrogen bonding and the molecular structural features that bring it about. Web if you were to draw every carbon hydrogen bond in organic chemistry class it would take you forever. Web so each oxygen is attached to 4 hydrogens, two are 1.01å covalent bonds and two are 1.75 å hydrogen bonds, and this results in a structure like figure 11.5.3, which has lots of void space, and the consequence that ice. Carbon is still bonded to these hydrogens but we're going to ignore them for our bond line structure. Web hydrogen bonding, interaction involving a hydrogen atom located between a pair of other atoms having a high affinity for electrons; The origin of hydrogen bonding. Be sure to learn them well and avoid them in your answers! Web there are various ways of drawing this and you will need to be familiar with all of them. Web amea is now turning its experience to the production of green hydrogen in egypt. The phosphate group, the deoxyribose sugar, and the nitrogenous base. Intermolecular forces (imfs) occur between molecules.
Diagram Hydrogen bond Molecule Water Chemical bond, oxygen bubble

Hydrogen Bond Definition and Examples
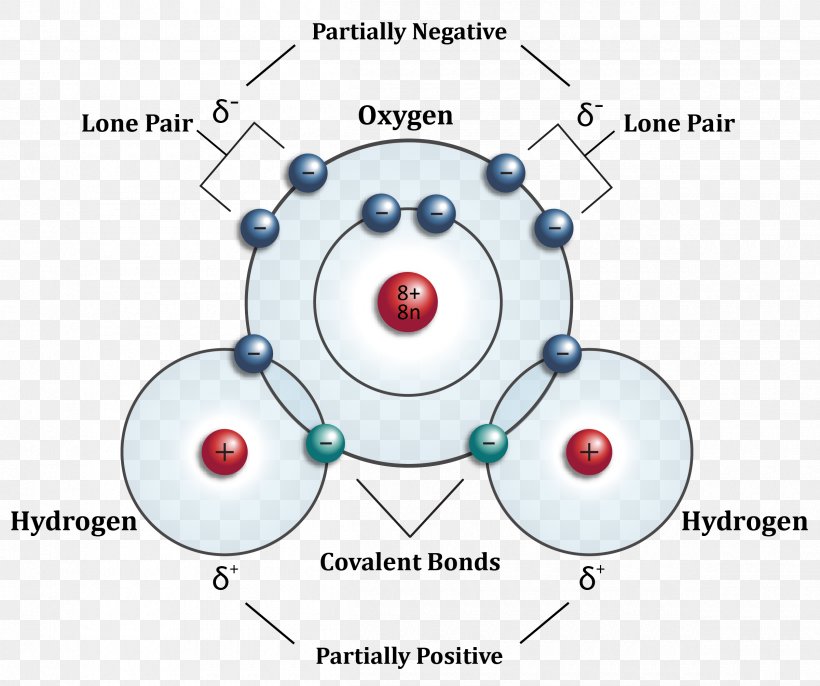
Hydrogen Atom Water Molecule Molecular Orbital Diagram, PNG
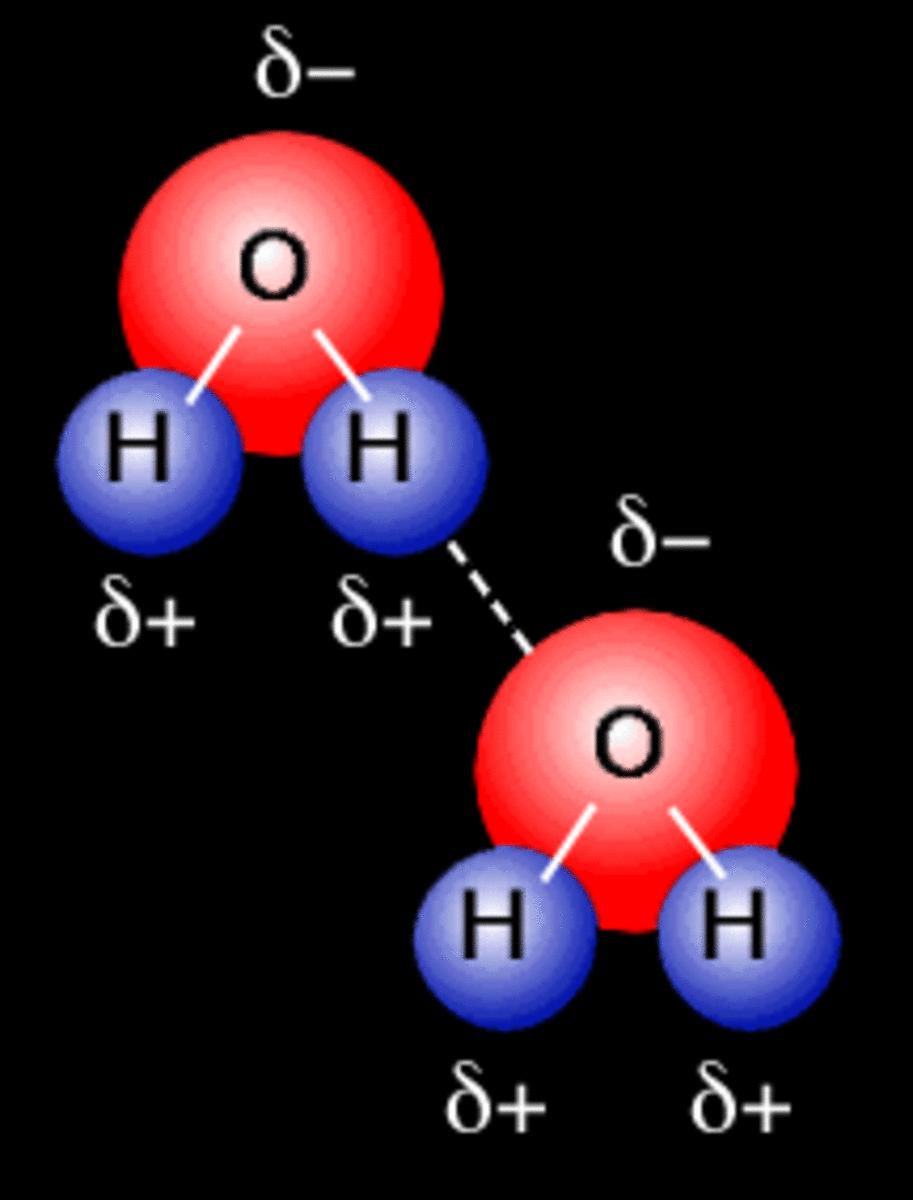
Primary and Secondary Bonds Owlcation

Hydrogen Bonding American Chemical Society

Hydrogen Bonding in water Dr. M. Chemistry Tutor

LabXchange
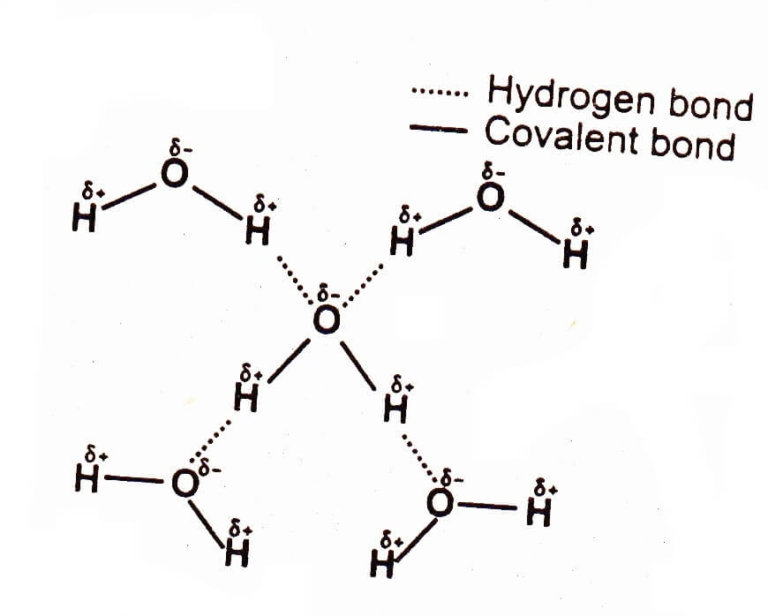
Hydrogen Bonding Chemistry Skills

Draw A Diagram Of Water Molecules Labeling The Hydrogen Bond And

H2O Lewis Structure, Molecular Geometry, and Hybridization
For Hydrogen Bonding To Take Place The Following Is Needed:
Web Water Molecules Forming Hydrogen Bonds With One Another.
Mr Khemistry Explains The Common Mistakes Students Make When Drawing Hydrogen Bond.
It Is Also Implemented As The Command.
Related Post: